Topic 4 - Intermolecular Forces and the States of Matter: Mastery
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
Intermolecular Forces
Additional forces (non-bonding attractions/repulsions between particles) that act when two substances are mixed
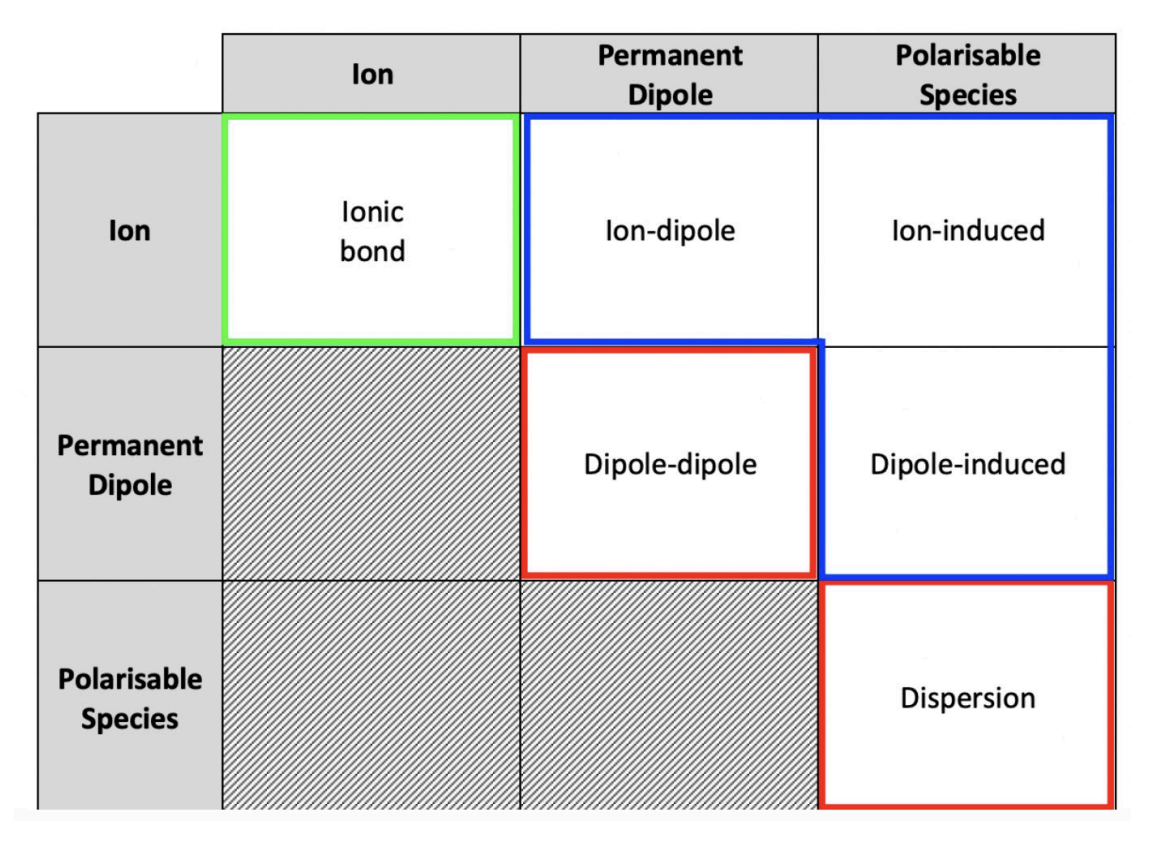
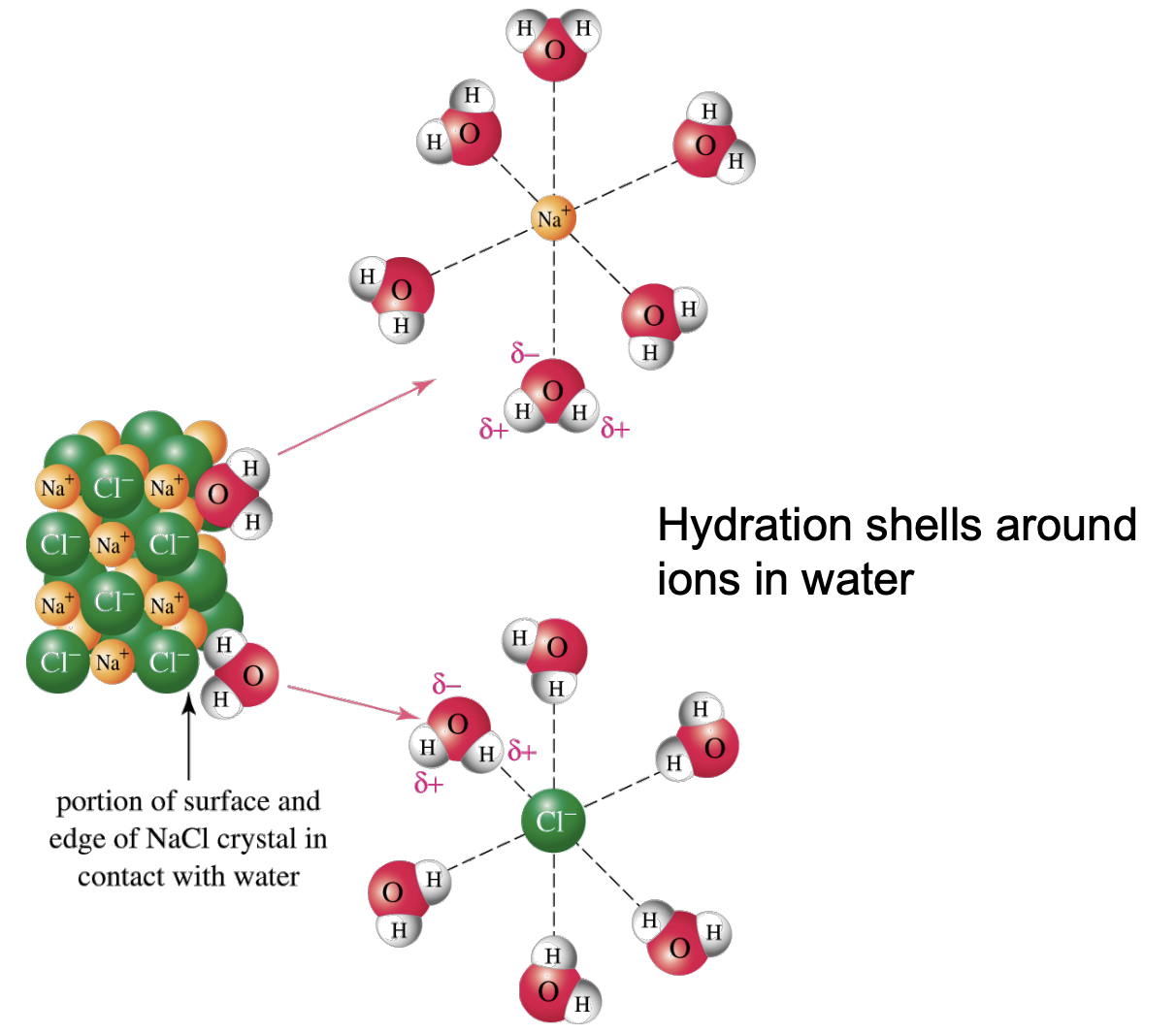
Ion-Dipole Interactions
Occur when dipoles align around an ion.
These are typically quite strong interactions.
They are very important for the solubility of salts.
Salts exhibit high solubility in polar solvents like water, but are insoluble in non-polar solvents like hexane.
Salt in Water: Hydration shells form around ions in water due to these interactions. The partial negative charge of a dipole aligns with a positive ion, and the partial positive charge aligns with a negative ion to create an attractive force
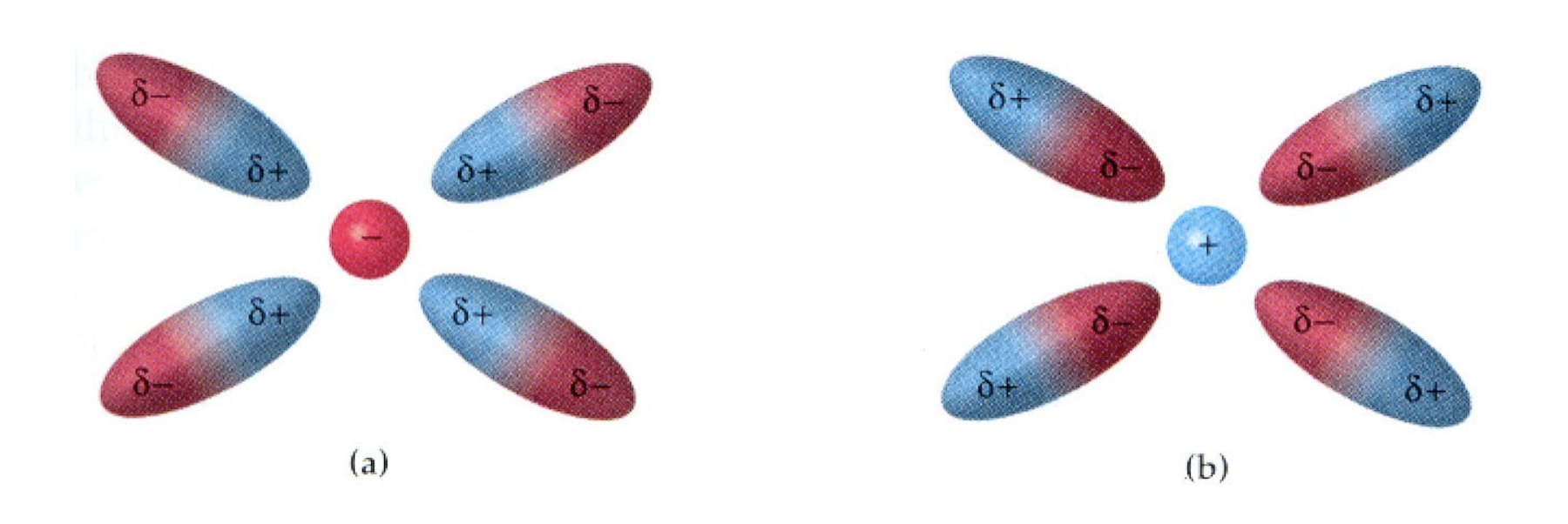
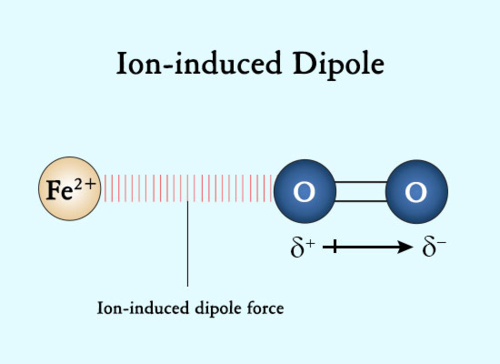
Ion-Induced Dipole Interactions
Similar to ion-dipole interactions, but they rely on an ion inducing a dipole in a non-polar species. The ion distorts the electron cloud, creating a temporary dipole.
They are much weaker than permanent dipole interactions.
These interactions are rare because ions typically do not dissolve in non-polar substances due to the salt's stronger ion-ion interactions.
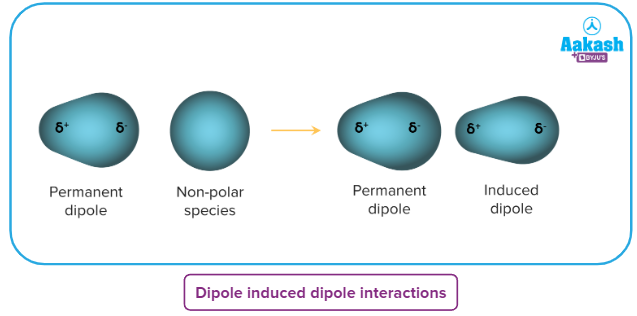
Dipole-Induced Dipole Interactions
Similar to ion-induced dipole, but these rely on the dipole in a polar species inducing a dipole in a non-polar species.
They are much weaker still.
Despite their weakness, they are important for all life.
A crucial example is the dissolution of non-polar oxygen in water, which is necessary for aerobic life.
General Attraction Forces: Dipoles
There is always an attraction between positive and negative charges.
Dipoles involve a nucleus and an electron cloud;
The negative electron cloud is attracted to the nucleus
The positive nucleus is attracted to the electrons
Solubility of Different Substances in Water
NaCl: High solubility (~6 mol/kg) due to ion-dipole interactions.
I2 (Iodine): Much lower solubility (~0.003 mol/kg) due to dipole-induced dipole interactions and van der Waals forces.
Oxygen: Even lower solubility (~0.0003 mol/kg) due to weaker dispersion forces and less ability to induce a dipole, but still crucial for life.
Sucrose (sugar): High solubility (~2 mol/kg or ~2 kg/kg) primarily due to hydrogen bonding and other interactions, as it is a very polar molecule with many -OH groups.
Ethanol: Entirely miscible due to extensive hydrogen bonding.
Hexane: Immiscible (~0.0001 mol/kg) with water, primarily interacting via dipole-induced dipole interactions
Identifying IMFs in Larger Molecules
Instead of treating large molecules as a single entity, it's helpful to break them down into components and consider their symmetry.
Solutions
Form when IMFs allow complete dissolution, resulting in a homogeneous mixture of single molecules or small clusters

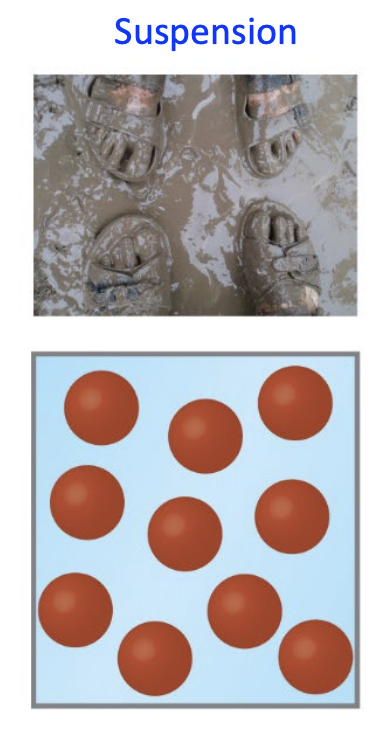
Suspension
Involve large solid particles that do not dissolve and is kept suspended by agitation; they will settle over time (e.g., mud)
Colloids
Consist of relatively large but tiny particles (e.g., <100 nm) that remain stable in suspension due to favorable surface interactions with the solvent
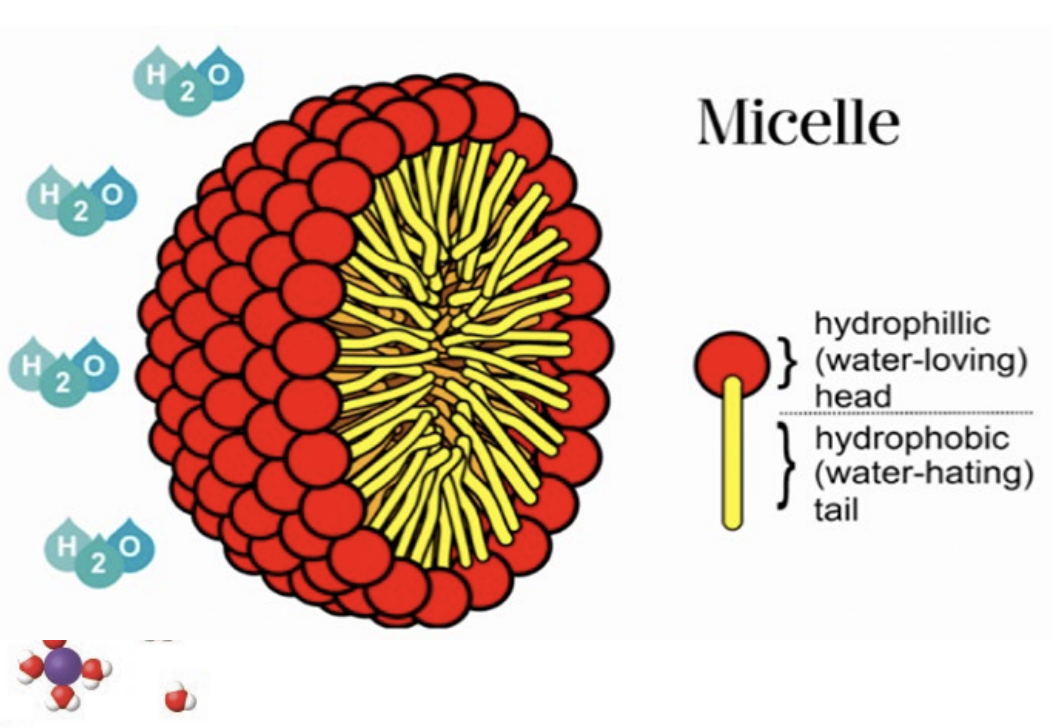
Micelles
Surfactants (like soap) are molecules with both a hydrophilic (water-loving) head group and a hydrophobic (water-hating) tail group
These molecules aggregate in water to form structures like micelles, where the hydrophobic tails are excluded from water and interact with other hydrophobic compounds (e.g., fats and oils), while the hydrophilic heads interact with water. This allows fats and oils to be encapsulated and washed away.
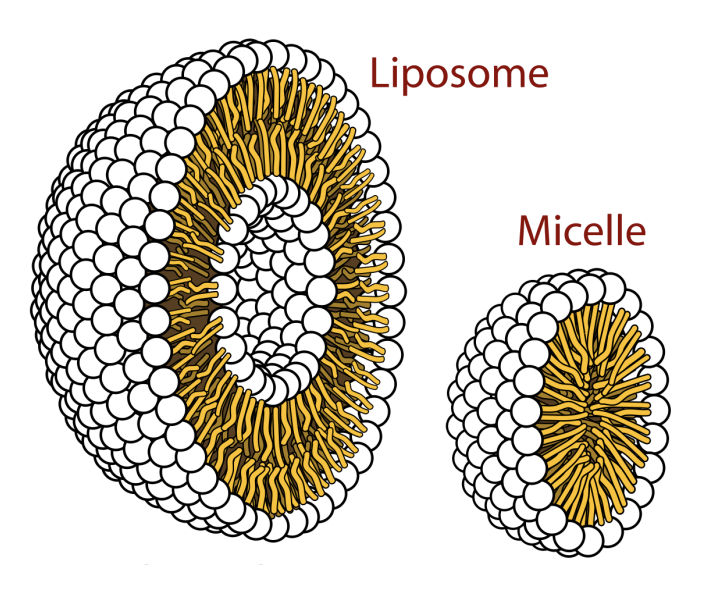
Liposomes
Double-layer spheres with both outer and inner hydrophilic cores, used for drug delivery.
Phospholipid Bilayer
Phospholipid bilayers form the majority of human cell membranes, separating two hydrophilic regions with a hydrophobic region
Amino Acids and Proteins
The structure of proteins are determined by inter- and intramolecular forces and is divided into four levels:
▪ Primary Structure
▪ Secondary Structure
▪ Tertiary Structure
▪ Quaternary Structure
Amino Acids and Proteins - Primary Structures
The sequence of individual amino acids held together by covalent peptide bonds. The nature of the R-groups (side chains) determines their polarity.
Amino acids are the building blocks of proteins.
They all share a common structure:
Amino group (–NH₂ or –NH₃⁺ in physiological pH)
Central carbon (the α-carbon)
Carboxyl group (–COOH or –COO⁻ at physiological pH)
Hydrogen atom (–H)
R group (side chain) → this is what differs between amino acids and gives them their unique properties (polar, nonpolar, acidic, basic, aromatic, etc.).
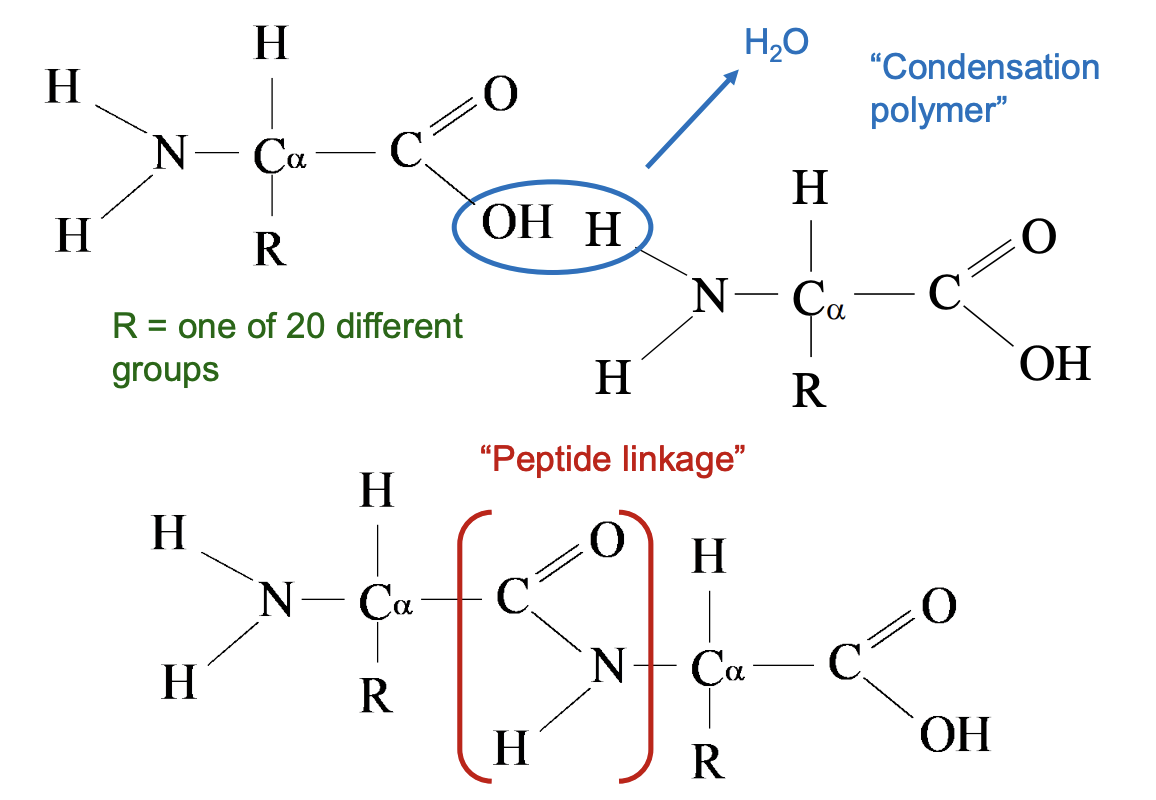
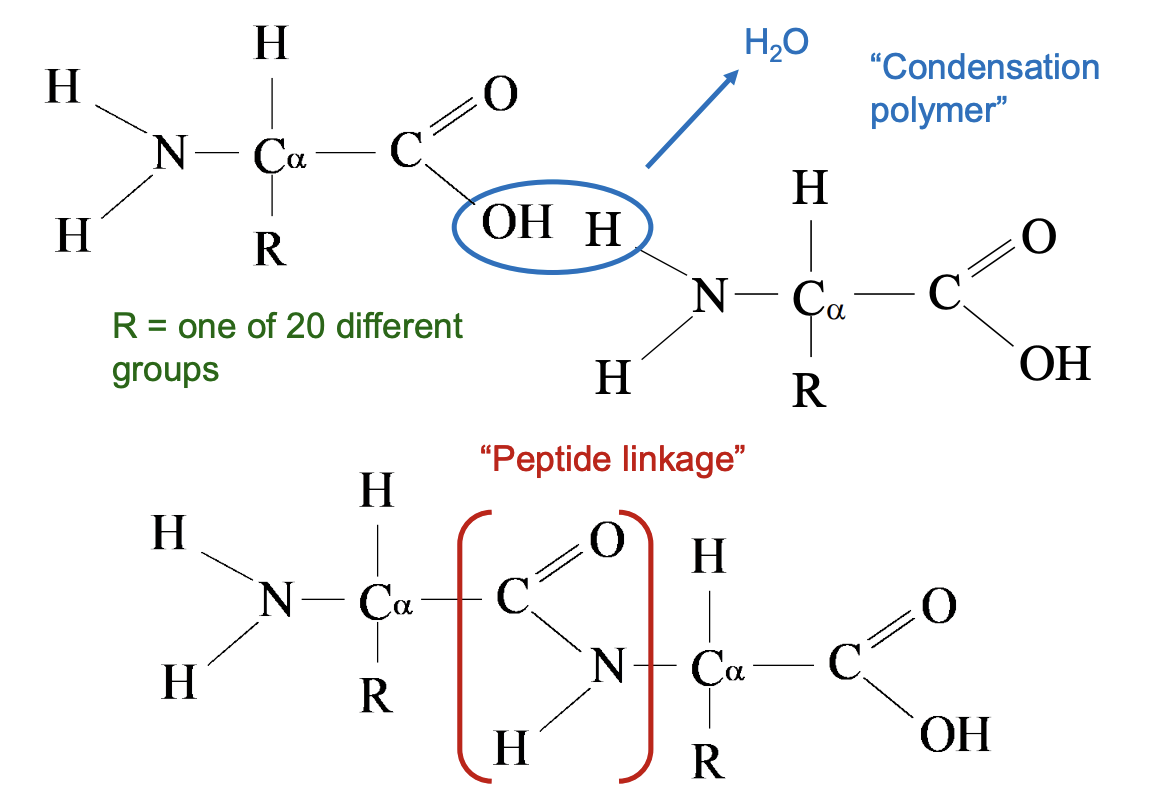
Amino Acids and Proteins - Primary Structures: Peptide Bonds
Peptide bond is a special covalent bond that forms between amino acids when building proteins.
It occurs between:
The carboxyl group (–COOH / –COO⁻) of one amino acid
And the amino group (–NH₂ / –NH₃⁺) of the next amino acid
A condensation reaction happens (a molecule of water is released).
The resulting bond is: −COOH + NH2− → −CO–NH − + H2O
In a Lewis structure, the peptide bond looks like a C=O linked to an –NH (an amide linkage).
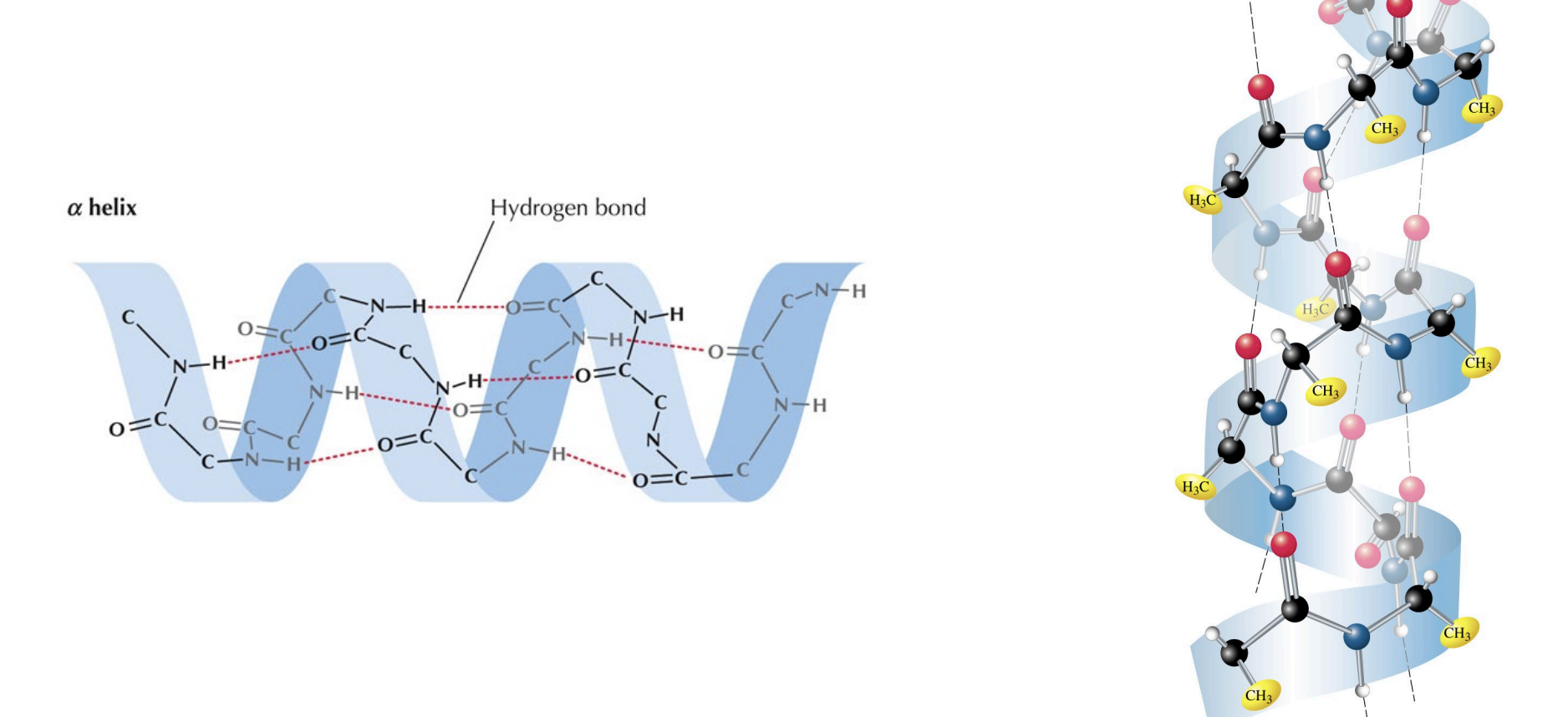
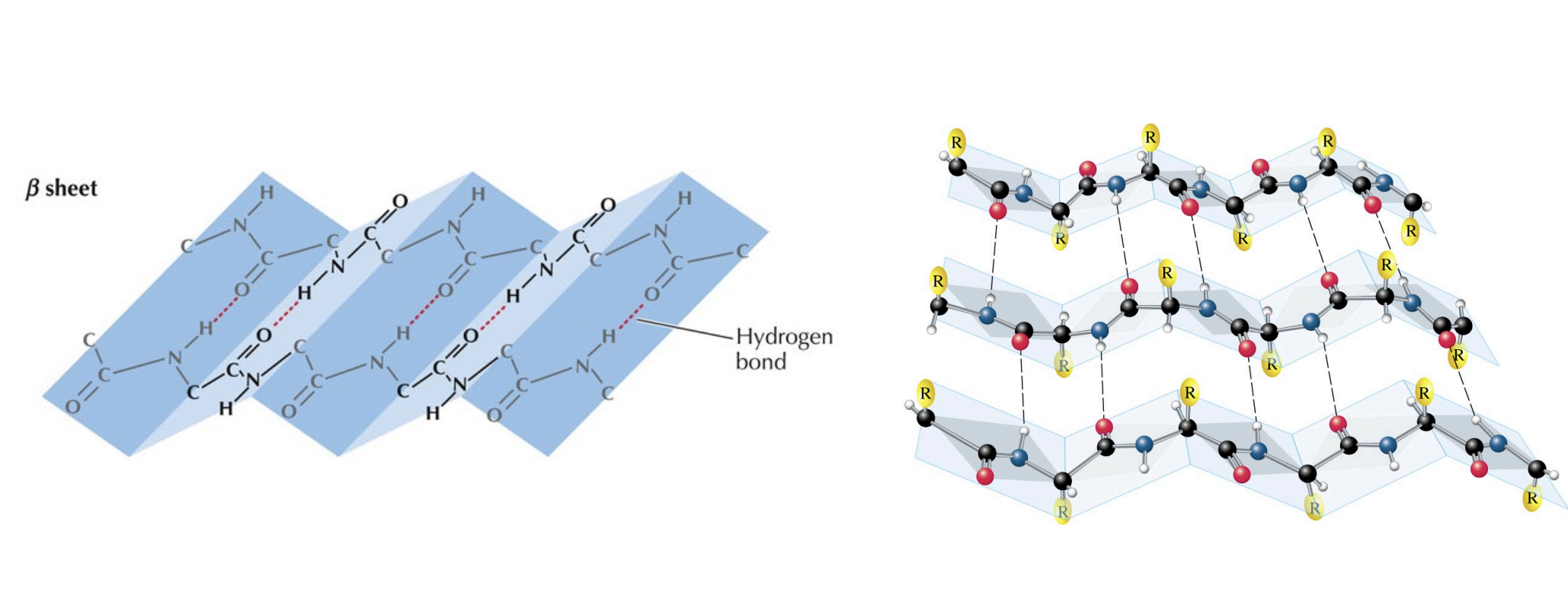
Amino Acids and Proteins - Secondary Structures
Small-scale structures, primarily alpha helices and beta sheets, are formed and held together by intramolecular hydrogen bonds between the C=O and N-H groups of the protein backbone
Amino Acids and Proteins - Tertiary Structure
The overall three-dimensional shape of a single protein molecule, resulting from various intermolecular interactions among the R-groups
Amino Acids and Proteins - Quaternary Structure
Involves the aggregation and interaction of multiple polypeptide chains
Viscosity
The tendency for a liquid to resist flowing.
◦ Affected by the strength of IMFs, molecular shape, and temperature.
◦ High viscosity liquids have stronger IMFs.
◦ Low viscosity liquids have weaker IMFs.
◦ Larger molecules with long, tangled chains tend to have higher viscosity.
◦ Flow requires molecules to be pulled apart and move through the liquid. For example, water flows smoothly, but honey (due to stronger IMFs and larger molecules) does not
Phases and Energy
Substances exist as solids, liquids, or gases because of the balance between intermolecular forces (which try to hold molecules together) and kinetic energy (which tries to break them free). Different materials change phases at different temperatures
Solid Phase
Atoms are held tightly together by strong bonds (inter- or intramolecular forces).
Possesses a rigid structure and is not free to move (flow).
The bonds must be strong enough to withstand the thermal energy at a given temperature
Liquid Phase
Atoms are held loosely together by bonds (inter- or intramolecular forces).
Molecules have enough energy to overcome some, but not all, intermolecular forces.
Has a non-rigid structure and is free to move around (flow). For example, liquid water has fewer hydrogen bonds per molecule than solid ice, allowing molecules to move
Gas Phase
Atoms/molecules are not held together by intermolecular forces. Intramolecular forces typically still hold the molecules themselves together.
Molecules have enough energy to overcome all intermolecular forces.
There is large space between molecules/atoms.
Has a non-rigid structure and is free to move around (flow), conforming to its container
Ideal Gas - Assumptions
No attractive force between atoms or molecules: The only interactions are elastic collisions.
The volume of the gas itself is negligible: Gas components are treated as point particles with no physical volume.
Ideal Gas Behaviour
Ideal gases behave similarly. The same amount (number of molecules) of gas at the same temperature and volume will have the same pressure. Changing volume has a similar effect on pressure, and viscosity is comparable at constant temperature and pressure
Gases behave ideally at extremely low pressures
pV/nRT = 1
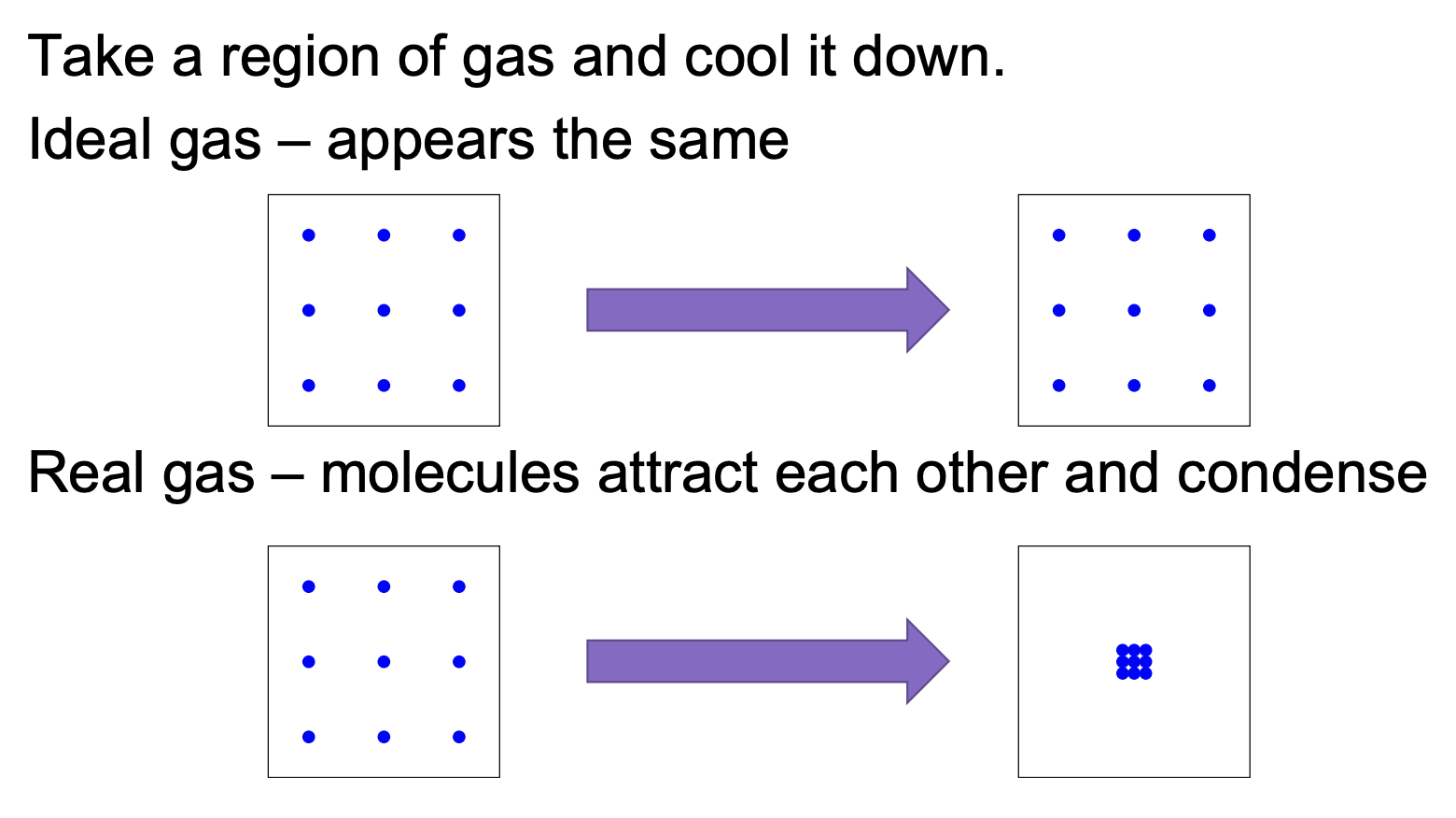
Real Gases
Deviate from ideal behavior because the ideal gas assumptions are not perfectly true
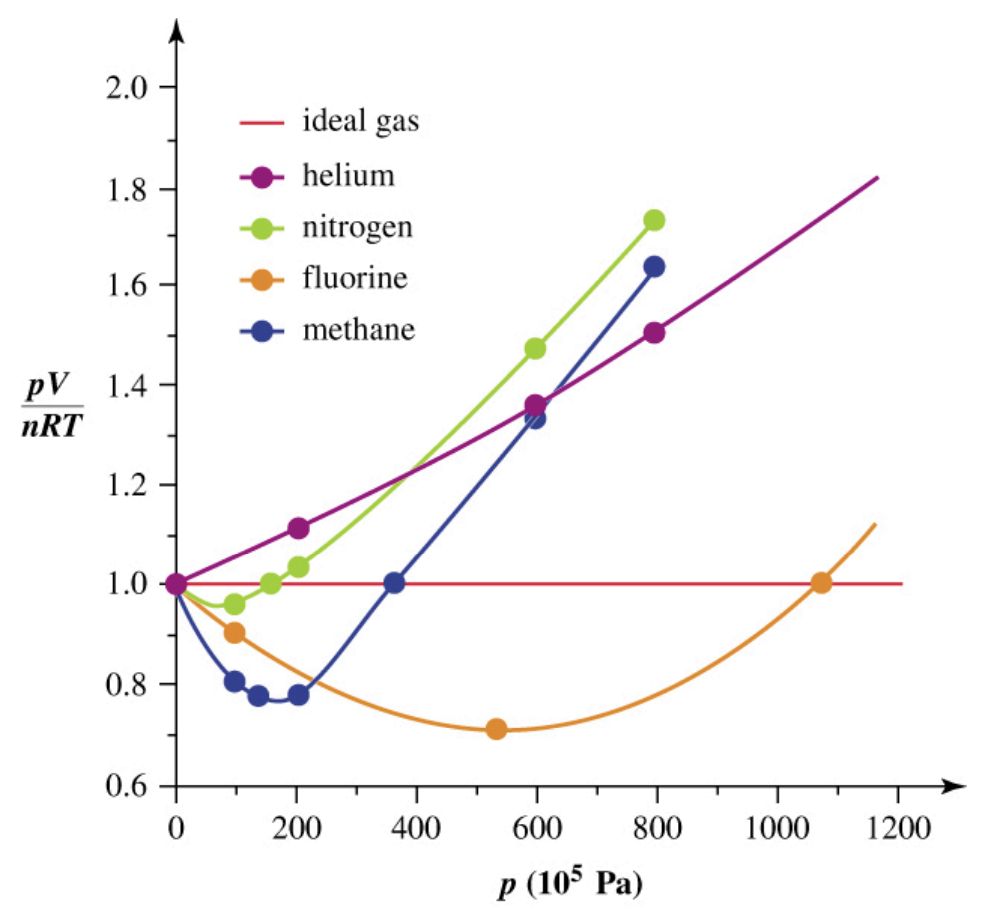
Real Gases - Deviations from the Ideal
Reasons for Deviation:
At intermediate pressures (below ~400 × 105 Pa, or 200 x 105 Pa for Cl2): The volume is less than expected (PV/nRT < 1)... because at moderate pressures, molecules are close enough to feel attractive intermolecular forces that pull molecules together → reducing the effective pressure (Preal < Pideal) → to balance the equation (PV = nRT), it shows up as a smaller volume (PV/nRT < 1)
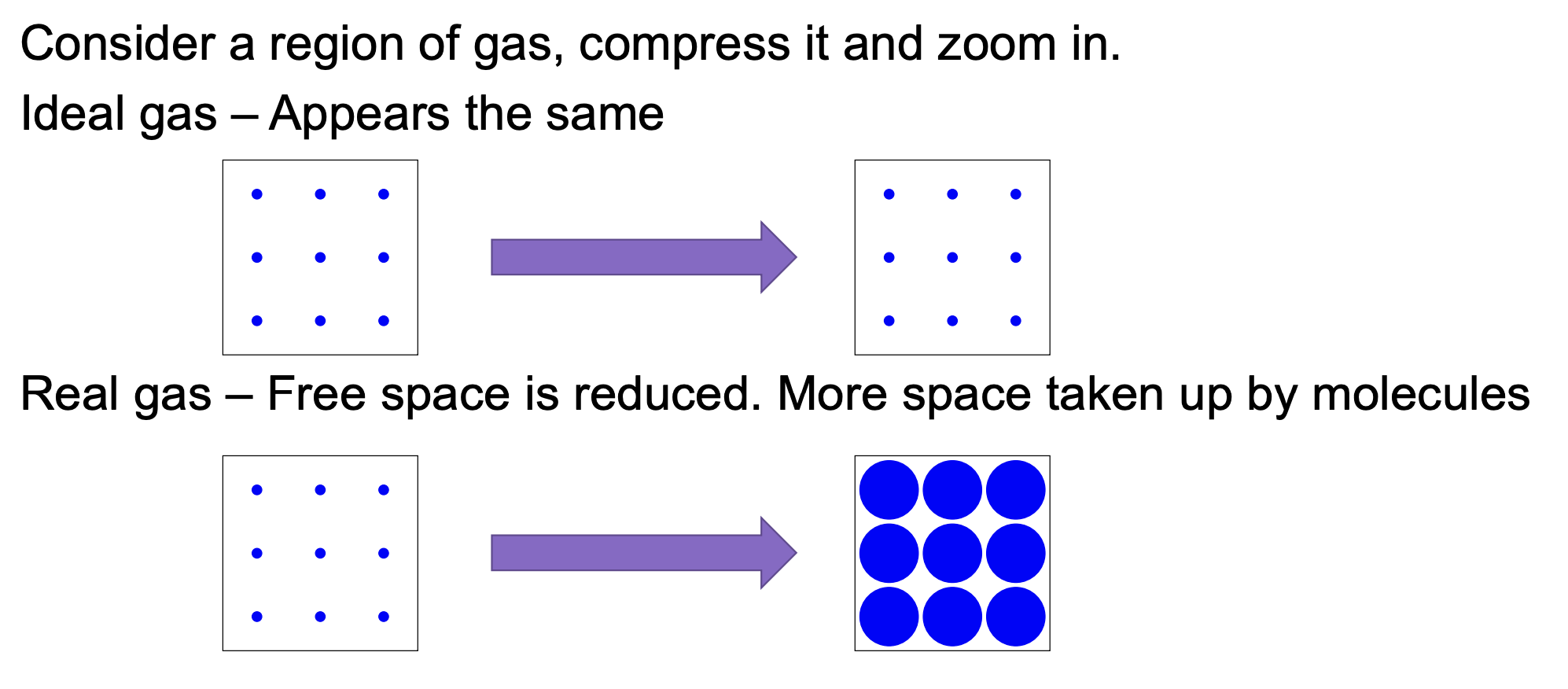
At high pressures (above ~400 × 105 Pa, or 200 × 105 Pa for Cl2): The volume is larger than expected (PV/nRT > 1)... because at very high pressures, molecules are forced close together → the finite size of the molecules themselves matters, they physically take up space, so there is less “free volume” for movement → this makes the measured volume larger than the ideal prediction (PV/nRT > 1)
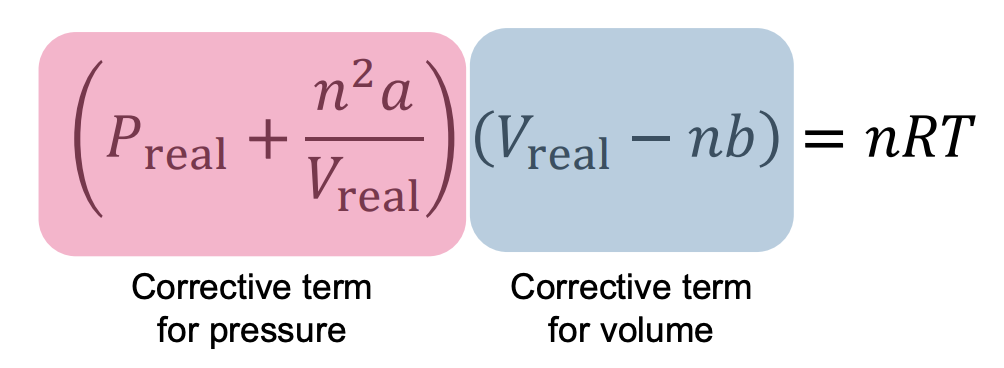
Van der Waals Equation
Johannes van der Waals proposed this equation to correct the ideal gas law for real gas behavior
The constant ‘a’ reflects the strength of the intermolecular forces. Stronger IMF means a larger ‘a’.
The constant ‘b’ reflects atomic/molecular volume. If they are larger then there’s a larger ‘b’ and less free space.
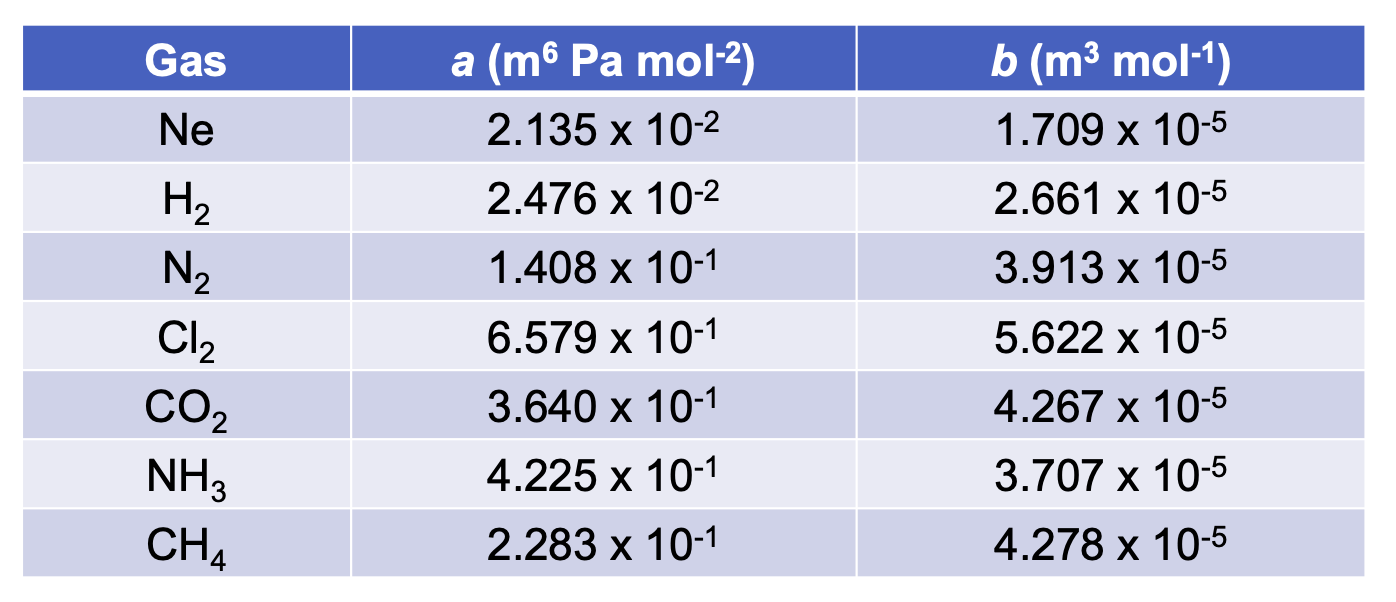
Phase Changes and Properties
Phase changes (melting/liquefying, boiling/vaporizing, sublimating) occur when enough energy is supplied to overcome intermolecular forces
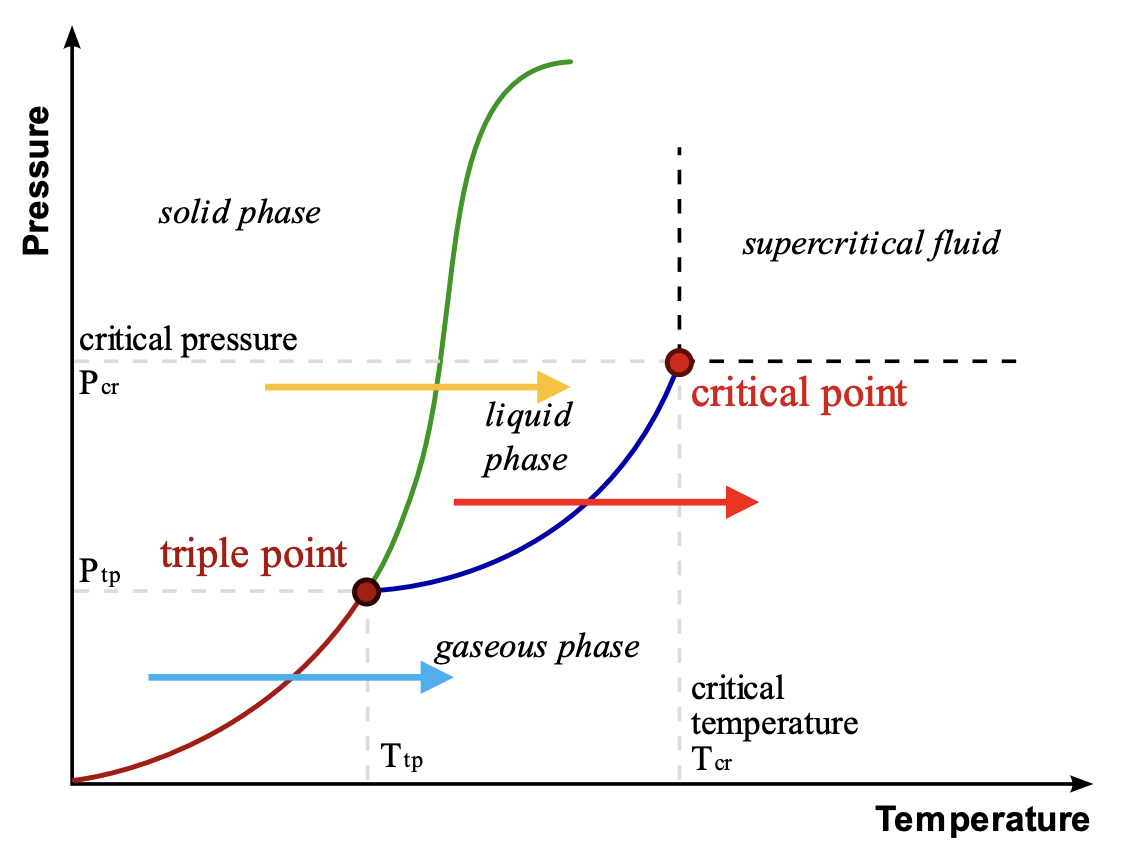
Enthalpy of Vaporization (ΔHvap)
Relates vapor pressure with boiling point
The energy required to overcome IMFs to convert a liquid to a gas.
As IMF strength increases → ΔHvap increases → vapor pressure decreases → boiling point increases
Boiling Point (BP)
The temperature at which the vapor pressure of a liquid equals the environmental (external) pressure.
The normal boiling point is specifically at 1 atm (1.013 x 10^5 Pa).
Boiling Point (BP) - Relationship with Intermolecular Forces
As IMF strength increases, vapor pressure decreases, and boiling point increase
Boiling Point (BP) - Affecting Factors
Molecular Size: For non-polar or similarly polar molecules, larger size leads to stronger dispersion forces and higher boiling points. Example: I2 > Br2 > Cl2 > F2.
Molecular Polarity (Dipole Moment): For similarly sized molecules, a larger overall molecular dipole leads to a higher boiling point due to stronger dipole-dipole interactions. Example: Acetonitrile has a higher BP than diethyl ether due to a larger dipole.
Hydrogen Bonding: Can significantly increase boiling points unexpectedly. For example, HF has a higher boiling point than other hydrogen halides (HCl, HBr, HI) because its strong hydrogen bonds require more energy to break, despite its small size. Generally, hydrogen bonding can overwhelm the effect of size unless the size difference is extremely large
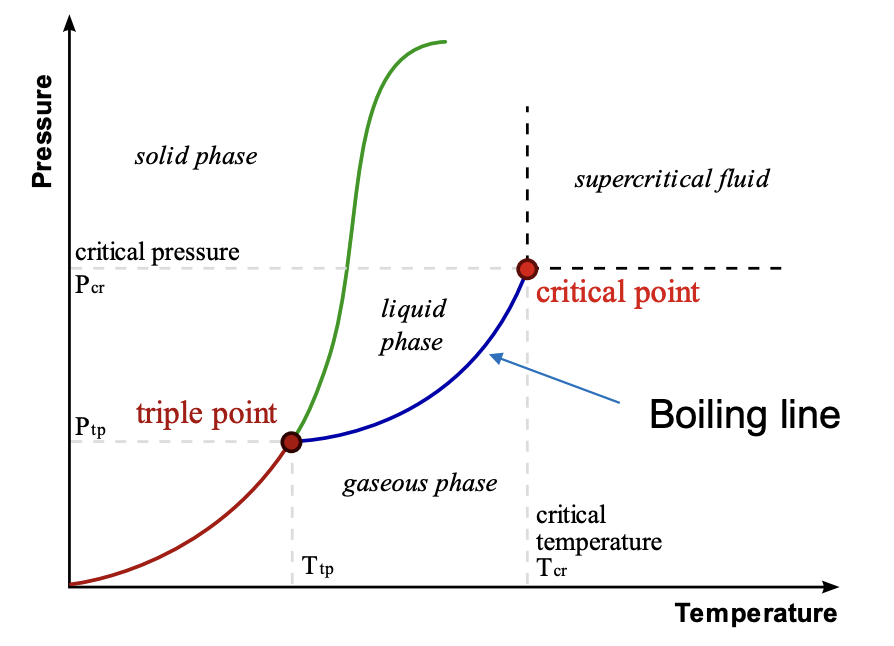
Melting Point (MP)
The temperature at which solidification and liquefaction are equally possible
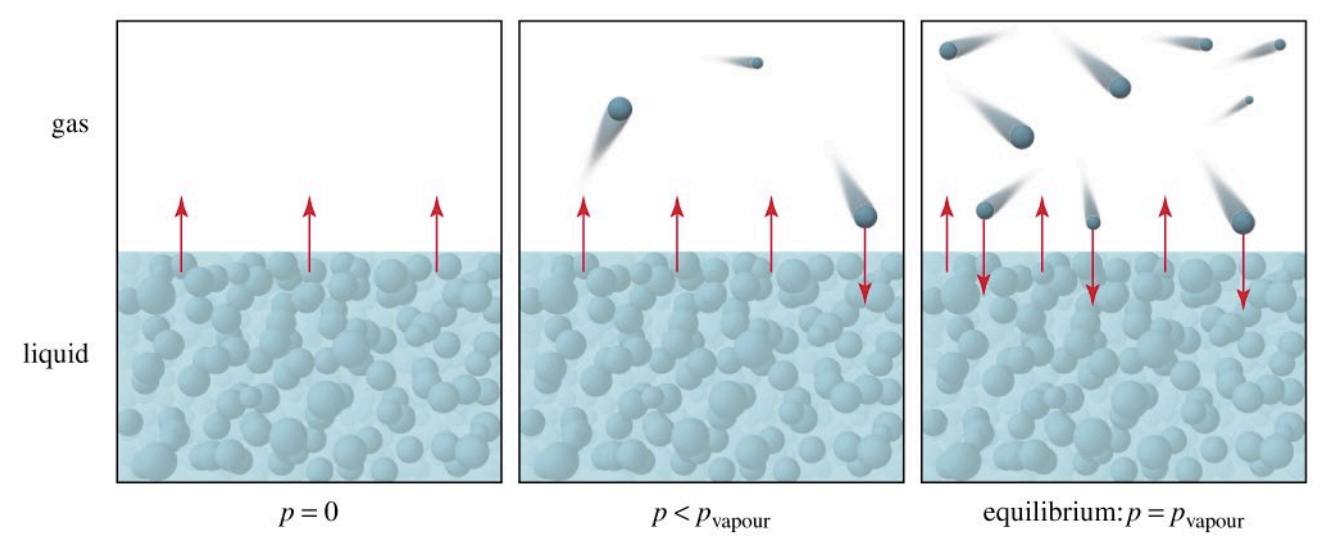
Vapor Pressure (VP)
The partial pressure exerted by a vapor in equilibrium with its liquid (or solid) at a given temperature, in a closed container.
Molecules constantly escape from the liquid phase into the gas phase, and gas molecules return to the liquid. Equilibrium is reached when these rates are equal.
VP depends on temperature and IMF strength, but not on the amount of liquid.
Higher temperatures increase kinetic energy, leading to more molecules escaping and higher VP.
Stronger IMFs reduce VP because more energy is required for molecules to escape.
The vapor pressure of a liquid is proportional to the 'a' constant from the van der Waals equation
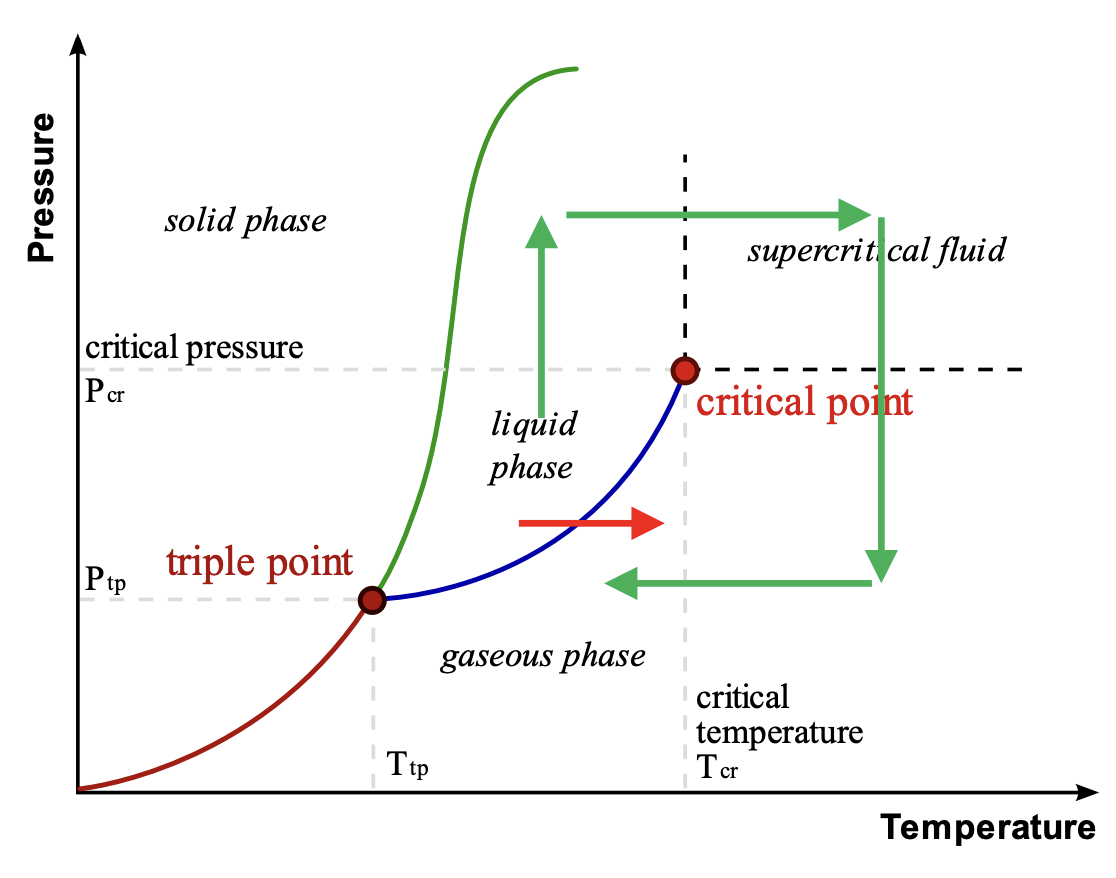
Supercritical Drying
A process used to dry delicate structures without damage. Conventional drying can cause shrinkage and collapse due to IMFs pulling the structure as liquid evaporates
Process: Involves starting from the liquid phase, pressurizing it above its critical pressure (Pcr), then heating it above its critical temperature (Tcr) (entering the supercritical fluid region), then lowering the pressure, and finally lowering the temperature. This allows the substance to transition from liquid to gas without crossing a phase boundary
Liquid Mixtures - Raoult's Law (for Ideal Mixtures)
In an ideal solution, the vapor pressure contribution of each liquid is “diluted” in proportion to how much of it is actually present.
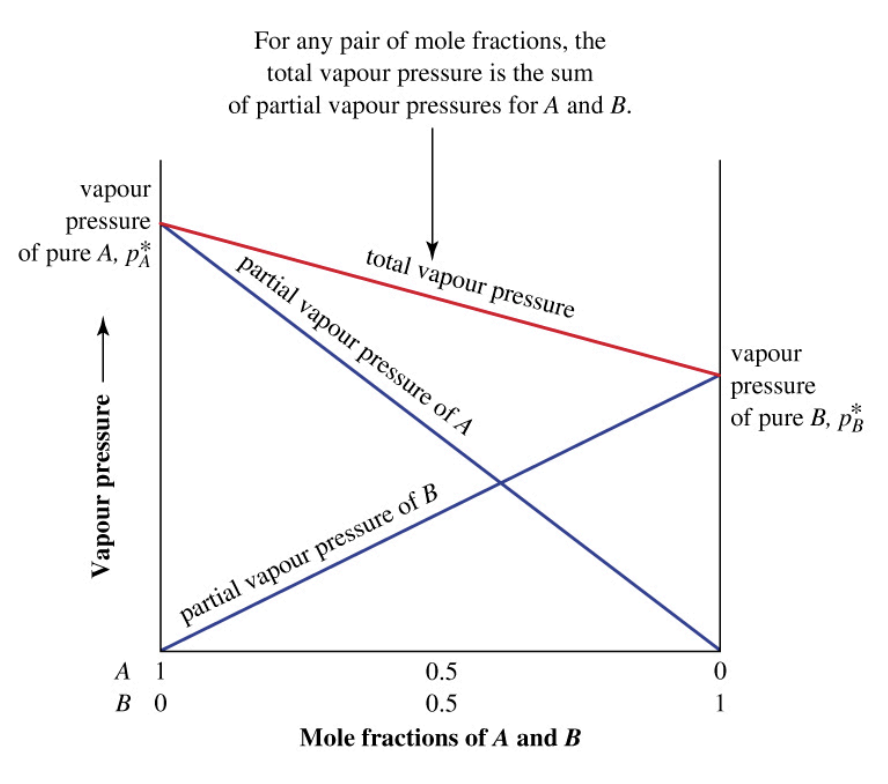
Liquid Mixtures - Raoult's Law (for Ideal Mixtures): Formula
Formula:
PA = χAP°A
Ptotal = χAP°A + χBP°B + ...
Where:
PA = the partial pressure due to A
χA = the mole fraction of A in the liquid phase
P°A = the normal vapour pressure of pure A
The total vapor pressure of the mixture is the sum of the partial pressures of its components
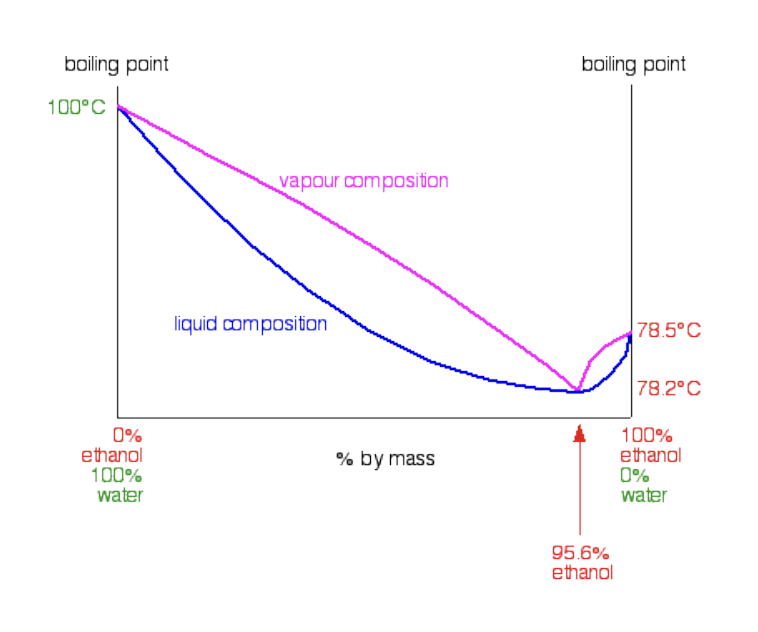
Liquid Mixtures - Raoult's Law (for Ideal Mixtures): Applications
In distillation, the component with the higher vapor pressure (more volatile) evaporates more readily.
This makes the vapor phase richer in that component.
By condensing the vapor, you can separate/purify it (like ethanol from water).
Liquid Mixtures - Raoult's Law (for Ideal Mixtures): Deviations from Raoult's Law
Not all solutions are ideal, meaning the intermolecular forces between components in the mixture are not the same as in the pure substances
Positive Deviation: Occurs when intermolecular forces between components (A:B) in a mixture are weaker than the forces within the pure components (A:A and B:B). This leads to a higher total vapor pressure than predicted by Raoult's Law. Molecules escape more easily.
Negative Deviation: Occurs when intermolecular forces between components (A:B) in a mixture are stronger than the forces within the pure components (A:A and B:B). This leads to a lower total vapor pressure than predicted. Molecules are harder to escape.
Azeotropes: Deviations can result in maximum or minimum boiling points on a phase diagram, creating an azeotrope where the liquid and vapor phases have the same composition, limiting the purity achievable by simple distillation
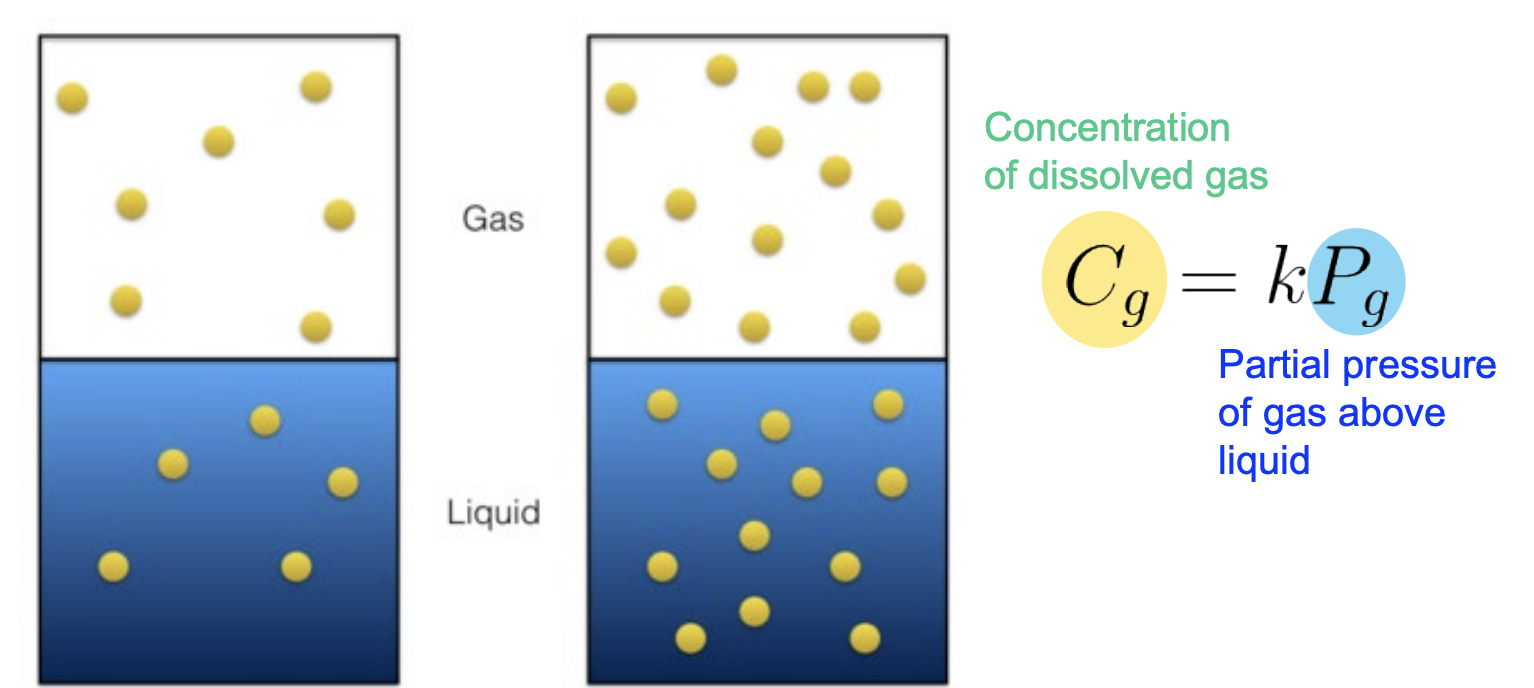
Henry's Law (Gas Solubility in Liquids) - Formula
At a constant temperature, the concentration (C) of dissolved gas is proportional to the partial pressure (Pgas) of the gas above the liquid
Formula: Cg = kH x Pgas, where kH is Henry’s Law’s constant
Henry's Law (Gas Solubility in Liquids) - Temperature Dependence
Gas solubility typically decreases at higher temperatures. This is because higher temperatures favor the gas phase, making it harder for gases to dissolve in liquids. In contrast, solubility of solids and liquids generally increases with temperature.
Henry's Law (Gas Solubility in Liquids) - Real Life Implications
Ocean Acidification: Increased atmospheric CO2 leads to more CO2 dissolving in oceans, acidifying the water and harming carbonate-dependent marine life.
Fish Deaths: Rising global temperatures decrease dissolved oxygen in water, which can lead to large fish mortality.
Surface Tension
The resistance of a liquid to increase its surface area, originating from attractive cohesive forces among molecules within the liquid. Molecules at the surface experience a net inward pull towards the bulk liquid because they lack balanced forces pulling outwards

Capillary Action
The phenomenon where a liquid in a narrow tube climbs the walls due to a balance between cohesive forces (holding liquid together) and adhesive forces (holding liquid to the solid). If adhesive forces are strong enough (e.g., water on glass), the liquid is pulled up against gravity
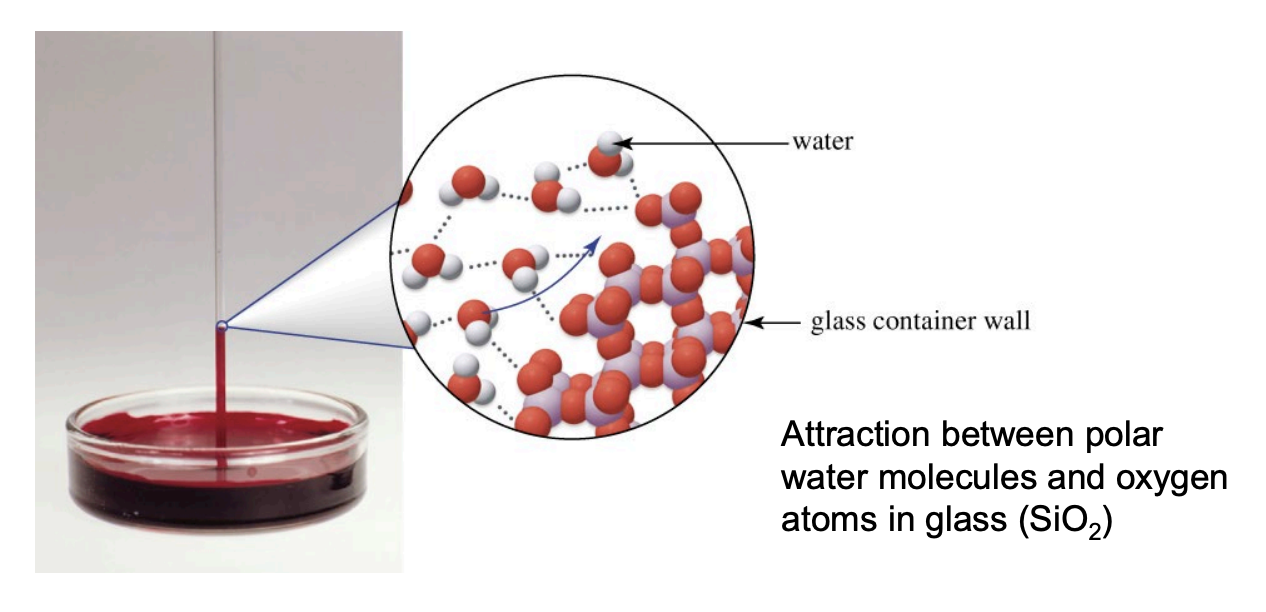
Meniscus
The curved surface of a liquid in a container, whose shape depends on the relative strengths of adhesive and cohesive forces
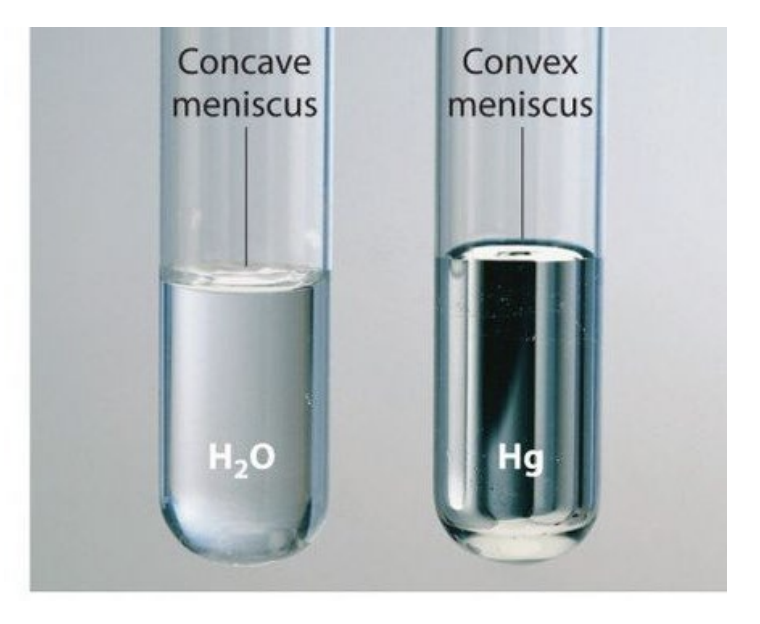
Meniscus - Convex Meniscus
Forms when cohesive forces are much stronger than adhesive forces. The liquid prefers to hold itself together and pulls away from the container walls, bulging upwards
Meniscus - Concave Meniscus
Forms when adhesive forces are equal to or stronger than cohesive forces. The liquid is attracted to and climbs the container walls, curving downwards
Cohesive Forces
Attraction between molecules of the same liquid (e.g., water–water via hydrogen bonding).
Adhesive Forces
Attraction between liquid molecules and the container surface (e.g., water–glass via hydrogen bonding).