Proton Therapy
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
43 Terms
How does the Bragg Peak form
As protons travel through a medium, their velocity is reduced. As this happens, the probability that a proton will interact increases. The result of this is that the dose deposition from a proton beam is concentrated near the end of their respective paths.
Generally describe how protons interact in tissue
stay in approximately straight paths
interactions function to slow down the proton and shorten its penetration depth
The rate of energy loss (i.e. the stopping power) is inversely proportional to the square of the proton’s velocity. Therefore, as the proton slows down, the stopping power greatly increases
Range of RBE for protons and what effect this
Range is about 1-1.8
With Linear Energy Transfer (LET) - the RBE increases with increasing LET.
Due to the Bragg Peak, the LET is highest near the end of a proton's range, therefore, having the highest RBE at this location (perhaps as high as 1.7).
Because many beams are used to form the Spread Out Bragg Peak (SOBP), these effects tend to average out to around 1.1 - 1.5.
With alpha/beta ratios - the RBE increases with decreasing alpha/beta ratios.
With dose - the RBE decreases with increasing dose.
At high doses, the RBE trends towards 1.0.
With genomic factors - the RBE increases due to suppressed repair mechanisms.
What RBE do we practically use and how can this be significant clincially
AAPM TG-256 recommends due to the relative uncertainties surrounding the exact RBE value and the complexity of implementing accounting for these differences to continue to use the value of 1.1.
The caveat to this lies with normal tissues that exhibit max point dose constraints at the end of the proton path (for instance, spinal cord immediately following the tumor). The RBE in this region could be quite high, and it is recommended to choose beams that avoid this scenario or reduce the allowed max dose to the structure.
How do you form a SOBP
Take multiple Energies - bragg peaks must be
Shifted in depth, AND
Weighted in order to form a correct SOBP.
Define Range straggeling
Range Straggling - Varying energy to change the depth of penetration.
Side-Note: The Bragg peaks for lower energy beams are narrower. This is a result of higher energies having longer paths. Due to the random nature of the interactions, some protons end up with slightly higher/lower energies. These differences are exaggerated over longer interaction histories resulting in higher energies having wider Bragg Peaks. This is a manifestation of range, or energy, straggling.
ICRU 78 definition of modulation
distance from the 90% distal to 90% proximal end
ICRU 78 definition of range
distance to 90% distal end
ICRU 78 definition of reference point distance
distance from surface to midpoint of modulation
ICRU 78 definition of distal penumbra
distance between 80% and 20% of the distal end of the SOBP
ICRU 78 definition of Distal End
distal 90%
ICRU 78 definition of proximal end
proximal 90%
ICRU 78 definition of field size
distance between 50% of lateral penumbra
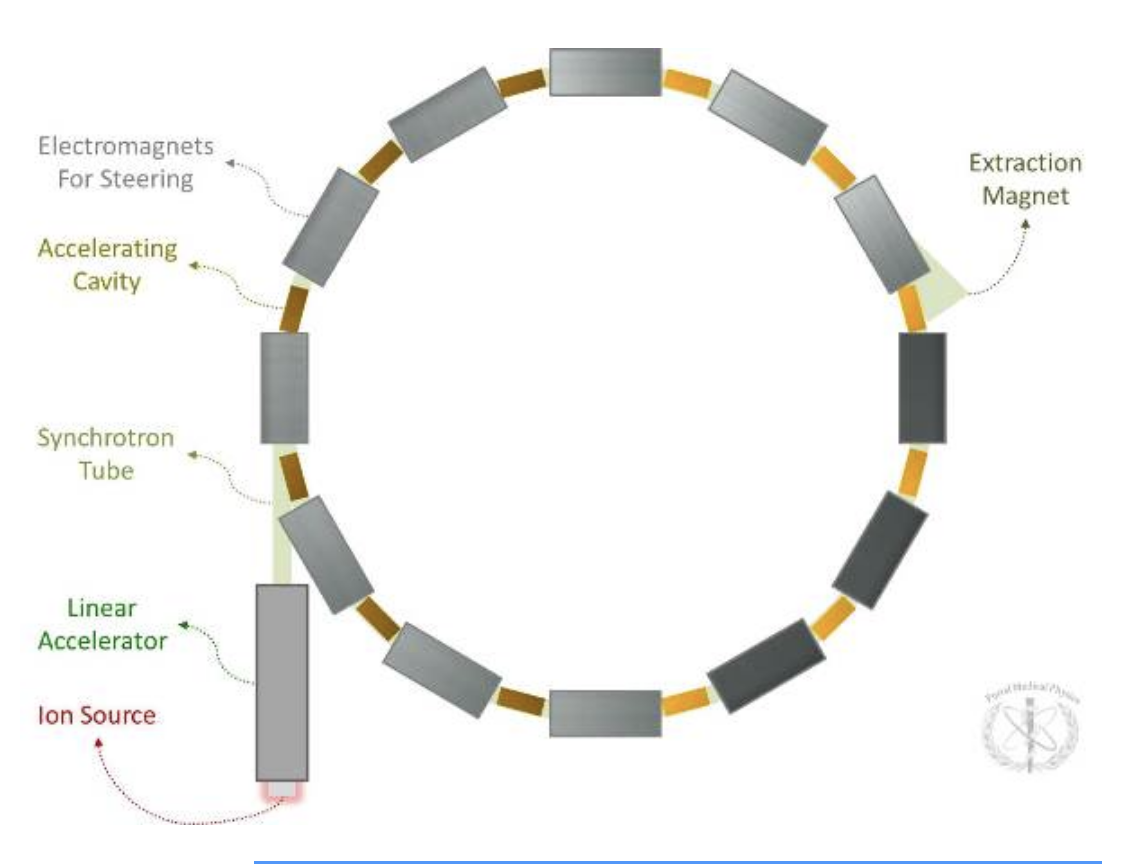
Explain and draw a synchotron
A cyclic accelerator for which the magnetic and electric fields are synchronized with the particle beam.
Magnetic and electric fields are varied (synchronous to particle revolution frequency).
Synchrotrons are commonly used in conjunction with linear accelerators. The linear accelerator provides the particle at high speed while the synchrotron acts as a “booster” accelerating the particle to its final energy. Synchrotrons cannot accelerate a particle from rest.
Because both the electric and magnetic fields are varied, the accelerated particles can achieve much higher energies as compared to the cyclotron.
Energies can be more easily varied (polyenergetic).
Because synchrotrons can easily vary energy, energy variation can be used to modulate depth control of the beam (instead of having to use range shifting with a physical variable thickness absorber).
Synchrotrons produce a pulsed beam because of the time required to cycle the magnets. (Beam off to beam on time is on the order of several hundred milliseconds to 1 second).
Typical diameter of synchrotron accelerator
~8-10 m (protons).
~25 m (carbon ions).
Explain and define a cyclotron
A cyclic accelerator for which the electric field is alternated as the magnetic field is kept constant.
Only the electric field is varied (while the magnetic field remains constant). The electric field is varied as a square wave with constant frequency changing the sign of the field (while keeping the magnitude of the field constant).
Two “dee” shaped hollow electrodes are kept under vacuum between magnetic upper and lower poles. The particles start at zero energy at the center of the magnet and spiral outwards gaining energy as they are accelerated by the rapid variation of voltage between the dees. The maximum energy is defined by the diameter of the revolution of the particle.
Because the magnetic field is kept constant, there is a fundamental limit on the size of the magnet (and therefore on the achievable energy for the accelerated particles).
Typically, monoenergetic.
Range is modulated by using energy degraders.
The disadvantage of this is that cyclotrons generally produce greater neutron contamination due to the necessity of the degrading materials (as compared to synchrotrons).
Continuous beam.
Typical diameter of cyclotron accelerator = ~3.5-5 meters (protons).
Explain and define a synchocyclotron
Another category of accelerator that is compact enough to mount directly on the gantry avoiding the need for a complicated beamline.
Design is similar to a cyclotron except that the frequency of the electric field is varied across the gap so as to account for relativistic effects.
Due to this frequency variation, it can only accelerate a single bunch of ions at a time. Once those exit the accelerator, a new bunch can be accelerated. The result is a pulsed beam.
Can supply a single gantry/vault with beam and be mounted on the gantry.
Currently confined to a fixed highest energy output that is then scattered and degraded to the necessary beam parameters. Or perhaps a few energies with a slow change in energy possible.
A similar device that attempts to compensate for relativistic effects is the isochronous cyclotron. For this machine, the magnetic field increases with radius (as opposed to varying the electric field frequency).
What ancillary equipment is needed for proton therapy beam delivery
Energy Degrader:
Carbon wheel with variable thicknesses.
Decreases the energy level of the beam to the target maximum.
Produces a lot of neutron contamination.
Beam Line:
Under vacuum.
Bending magnets deflect beam.
Nozzle:
Contains either:
Scatterers (scattered beam) or,
Steering magnets (scanning beam).
Ionization chambers.
Snout holds:
Aperture and/or range compensator.
Explain the scattering method of proton delivery
Was traditionally the most common method.
Also referred to as passive scattering, capable of providing a circular or rectangular beam.
Narrow proton beams are spread out to a beam diameter on the order of 10-20 cm.
Collimators are then employed to shape the spread-out beam.
A bolus material is used in the depth direction to shape the beam in the beam direction.
Single Scattering - A single scattering medium can be used to broaden a proton beam for smaller fields.
Double Scattering - For larger fields, a dual scattering system is needed to get a uniform dose profile.
Explain the spot scanning method
Spot scanning Method (or pencil-beam/raster scanning):
Scanned beam techniques are beginning to look to be the accepted route for the development of future devices.
Uses “wobbler” magnets and particle energy selection to deposit peak within individual voxel-based targets.
The synchrotron adjusts the proton energy thereby adjusting the penetration depth.
Scanning electromagnets act as a beam position controller.
The treatment volume is effectively divided into many small volumes which are treated with individual proton beams (as defined by energy and position).
Can adjust the SOBP on a spot-by-spot basis.
Allows for Intensity Modulated Proton Therapy (IMPT) by raster scanning (i.e. “dose painting”) beamlets within voxels.
Neutron scatter is reduced compared with passively scattered beamlines.
Scanning the target area multiple times improves dose uniformity.
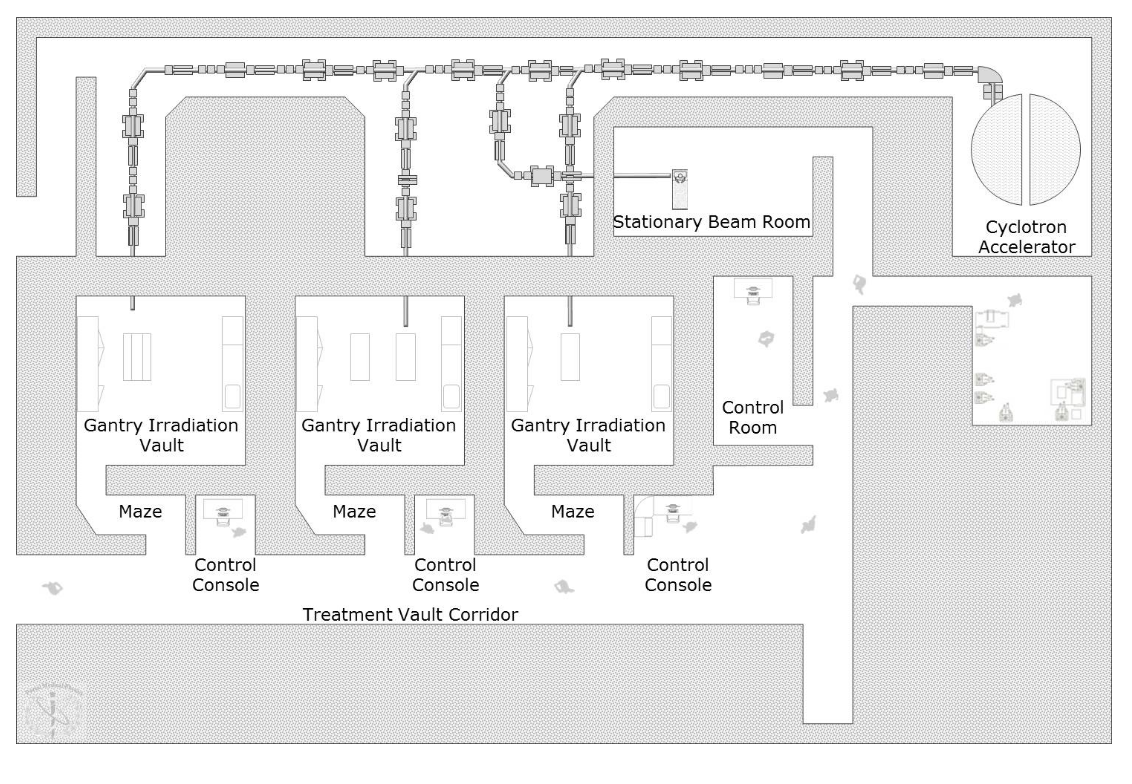
Draw a typical proton facility layout
Planning variables for proton therapy
Number of Beams.
Angle/Orientation of Beams:
Generally, the beam orientations for Single-Field Uniform Dose (SFUD) are selected such that the shortest distances from the surface to the target are achieved.
For Multi-Field Optimization (MFO), beams are orientated such as to minimize overlap to reduce skin dose.
Beams should not, in general, be pointed towards critical structures (due to uncertainties in the range of the beam).
SOBP Field Margins:
Range (depth of “useful dose”).
Modulation (width of “useful dose”).
Aperture Size.
historical dose regimens for ocular melanoma and low stage prostate
Some typical and/or historical dose regimes:
Ocular Melanoma: 14 GyRBE x 5 to 70 GyRBE.
Low-Stage Prostate Cancer: 1.63 GyRBE x 46 to 75 GyRBE.
SFUD vs IMPT
SFUD:
Treatment plans typically utilize multiple fields.
Individual (single) fields are optimized to achieve a more or less homogenous dose to the target.
Forward planning for proton therapy.
Intensity Modulated Proton Therapy:
All fields are optimized so they deliver a varied dose per field but when combined result in a homogenous dose to the target.
Also called Multi Field Optimization (MFO).
Inverse planning for proton therapy.
3 categories of proton dose calcs
Convolution/Superposition algorithms:
Operates similarly to this category of algorithm for photons.
Dose to a point is found by:
Summing dose from pencil beam kernels (precalculated using Monte Carlo), as these kernels are superimposed on a calculation grid.
Heterogeneities are taken into account by scaling the kernel dose distribution by electron density differences.
Pencil beam algorithms:
Involve a large number of finite small beams weighted according to the fluence.
Monte Carlo algorithms:
Gold standard, most accurate approach.
Individual protons are tracked through the calculation medium.
Likelihood of interactions are sampled using random numbers.
To achieve sufficient accuracy, millions of histories must be calculated.
This makes these calculations burdensome.
Describe the function of bolus as well as how the construction is done
Primary Function - Range Compensator.
Milled from polyethylene or wax (should be of low-atomic number).
The shape is matched to the distal shape of the target volume compensating for patient surface irregularity and internal heterogeneities (allowing for motion-effect, alignment errors, and other uncertainties).
In general who is the best candidate for proton therapy
Pediatrics - dose still developing
Patients with diseases that respond to dose escalation.
Treatment goal: the dose escalation is expected to provide a higher probability of local control (i.e. higher cure probability).
Examples:
Uveal melanoma
Unresectable sarcomas
Patients with diseases where increased precision is needed to reduce dose to normal tissues.
In this case, the doses are comparable to conventional radiotherapy.
Goal: Since there isn’t dose escalation, no increased probability of local control is expected; however, it is expected that side effects can be reduced.
Examples:
Prostate cancer
Medulloblastomas
Target motion - describe the issue and solution
Target miss can occur if tumor motion is not taken into account.
Margination can be utilized to take this motion into account and ensure that the tumor is being treated during all of its phases of motion (i.e. as in the Internal Target Volume).
Solution: cover all geometric positions of target.
Heterogeneity motion - describe the issue and solution
Target miss can also occur if the density of the patient geometry outside of the target and upstream of the beam is changing as a function of time.
Solution: use range adapted margins. Range adapted margins use a higher effective energy in regions behind a dense heterogeneity or in the regions where there may be a dense heterogeneity. The result is increased tissue being treated downstream of the target, but the target remaining in the beam.
Interplay effect - describe problem and potential solutions
For PHOTON beam delivery, this effect comes into play during IMRT when MLC motion is occurring at the same time as target motion.
For PROTON therapy, the two independent timelines that result in this effect manifesting are:
Target motion
Beam delivery timeline
If not reconciled, this can result in dose inhomogeneities (both cold and hot spots) within the target volume.
This effect is very strong on rastered beams but is basically removed for passively scattered beams as the beam is, in essence, using fixed modulation in the form of a compensator.
SFUD vs IMPT for motion
Single Field Uniform Dose (SFUD) versus Intensity Modulated Proton Therapy (IMPT):
For SFUD treatments, blurring will occur; however, each field will see a little different motion and the effect will be minimized.
For IMPT treatments, are much more susceptible to motion effects
What are the main controversies around Proton therapy
No controlled trials to show improvement and much more expensive'
Pressure to fill slots at facility to recover the investment but to do this have to use sites that dont really need it.
List the dosimetric benefits of proton therapy
There is considerably higher integral dose to normal tissues with photon external beam therapy compared to proton therapy.
There is no dose downstream from the target.
Dose deposition is maximum at the intended target depth.
It is notable that the bigger the field, the larger the advantage from protons in that the volume of normal tissue sparing increases. Protons, historically, have been associated with the treatment of small targets but this advantage highlights the possibilities of proton therapy for larger targets.
In general, proton beams demonstrate very little lateral penumbra as compared to photon beams.
The exception to this is that entrance skin dose is higher for proton therapy.
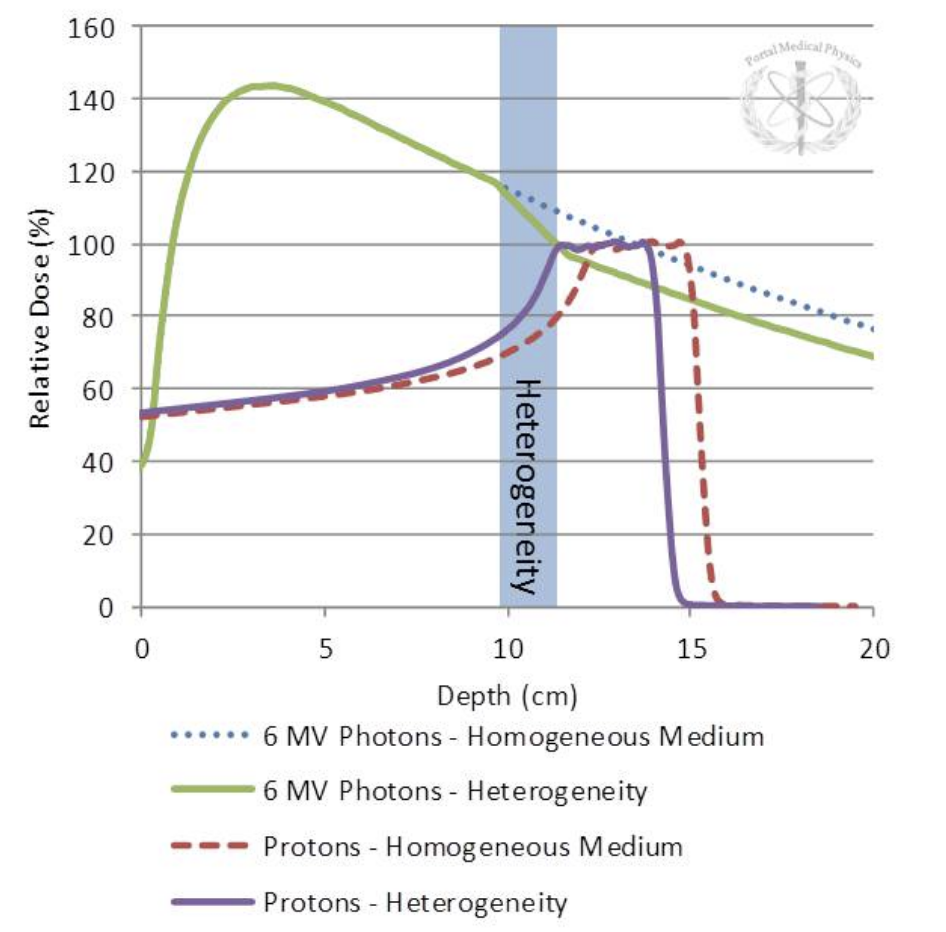
How do heterogeneities effect protons over photons - draw PDDs for with and without
Unlike photons, the presence of a denser inhomogeneity upstream from a proton beam will result in a reduction in energy (and, therefore, range of a beam) but not the intensity. There will also be some scatter of the beam.
3 main components of proton therapy QA
Beam Parameters
Measuring Output Factors
Measurements Flatness & Symmetry
Image Guidance
Isocentricity Test - Colinearity Test - Imaging Iso matches Beam Iso
Robotic Positioning System
List daily proton QA
Imaging
Output
Range Check
Dose per Monitor Unit
Spot Position
Reviewed once per week by a physicist
Software:
Communication
Couch isocentricity checked daily or weekly
List monthly QA tests
Proton-image isocentricity
Flatness and symmetry
Ranges and modulations
Mechanical
List Annual proton QA
PDDs and modulations
Combinations of field sizes and gantry angles
X-ray source and detector image characteristics
Dose rate dependencies
theoretical benefits/uses of PET Proton therapy
Detect daily setup differences.
Infer information on the actual proton therapy delivery and beam range in the patient.
Detect anatomical changes (the introduction of an unaccounted for heterogeneity can have a significant impact on proton radiotherapy).
In vivo dosimetry.
List the common proton therapy byproducts used for PET monitoring
O-15 (Half-Life = 2 minutes).
N-13 (Half-Life = 10 minutes).
C-11 (Half-Life = 20 minutes).
Shielding considerations for protons
The primary beam is not of great shielding concern as the protons would be effectively stopped within the patient.
Secondary radiation shielding must be attenuated to appropriate levels where secondary radiation refers to radiation produced from the interaction of the primary beam with any material that the beam is incident upon and can be:
Only present when the beam is on (prompt radiation).
Remaining after the beam is turned off (residual radiation).
One of the most notable products of proton interactions with materials is neutrons.
The yield of neutrons increases with proton energy.
Capture gamma rays are also present and can contribute to dose in mazes (in addition to neutrons).
sources of proton secondary radiation
Secondary radiation occurs at:
The synchrotron or cyclotron.
During beam transport.
At the energy degrader.
In beam collimation devices in the nozzle.
draw a carbon vs proton PDD and list the RBE
carbon - 2.5
protons - 1.1
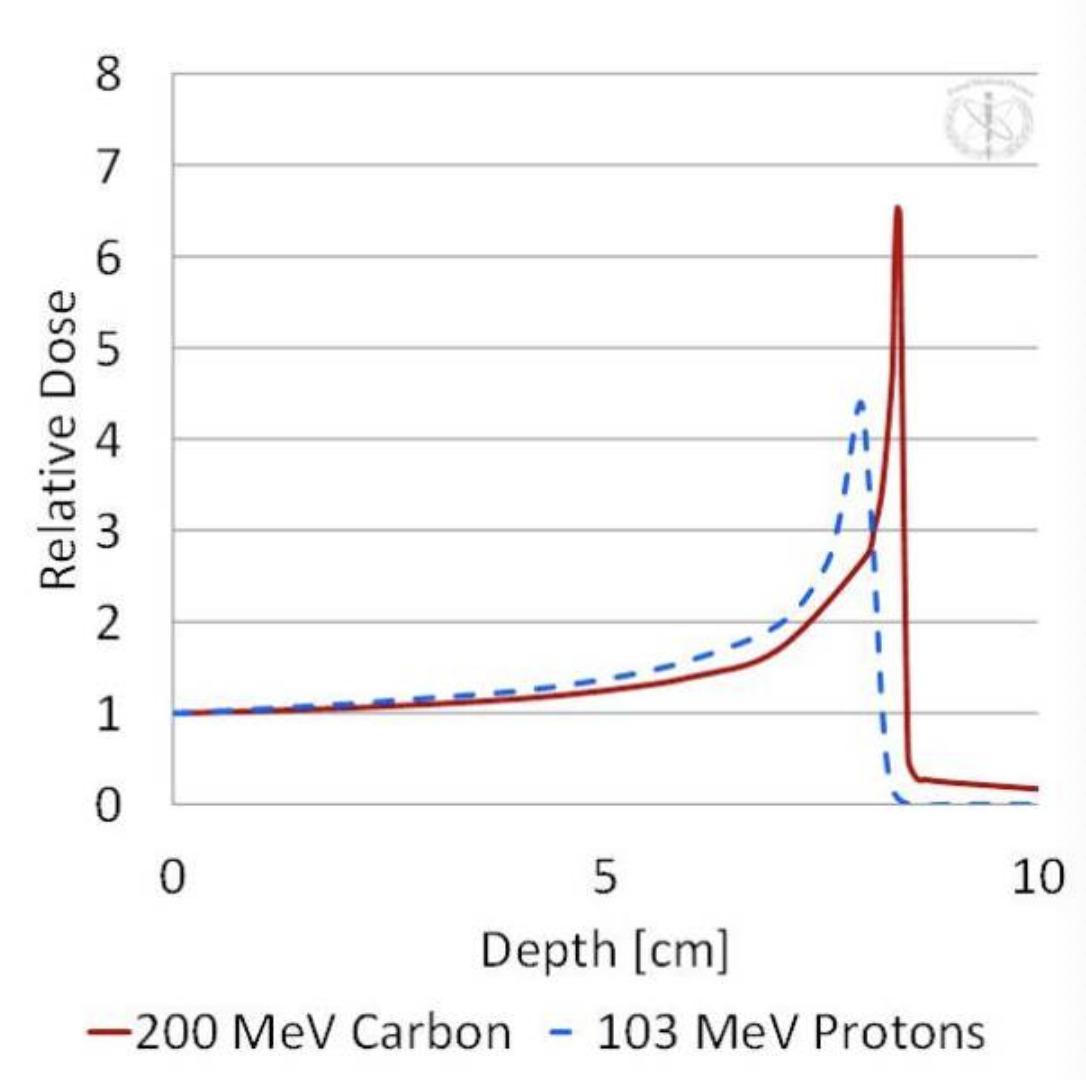
what is the reason for the sharper bragg peak in heavier ions
Electrons exhibit a much stronger range/energy straggling than protons. This is due to their much lower mass resulting in frequent trajectory deviations (a so-called “torturous” path). Compared to protons, carbon atoms have an even higher mass and, therefore, exhibit even less range/energy straggling. This results in a sharper and higher peak relative to protons.