Chemistry 1.11- Electrode Potentials and Electrochemical Cells
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
13 Terms
When is an electrode potential (E) established, and when is it positive or negative?
When a metal rod is placed in a solution of its own ions, it creates an equilibrium based on the tendency of the metal to lose electrons and be oxidised and the tendency of the ions to gain electrons and be reduced
Eg. Cu2+(aq) + 2e– ⇌ Cu(s)
A negative electrode potential is created due to oxidation (the positive ions enter the solution but the electrons accumulate on the rod), so the equilibrium position lies to the left
A positive electrode potential is created due to reduction (the positive ions gain electrons from the rod), so the equilibrium position lies to the right
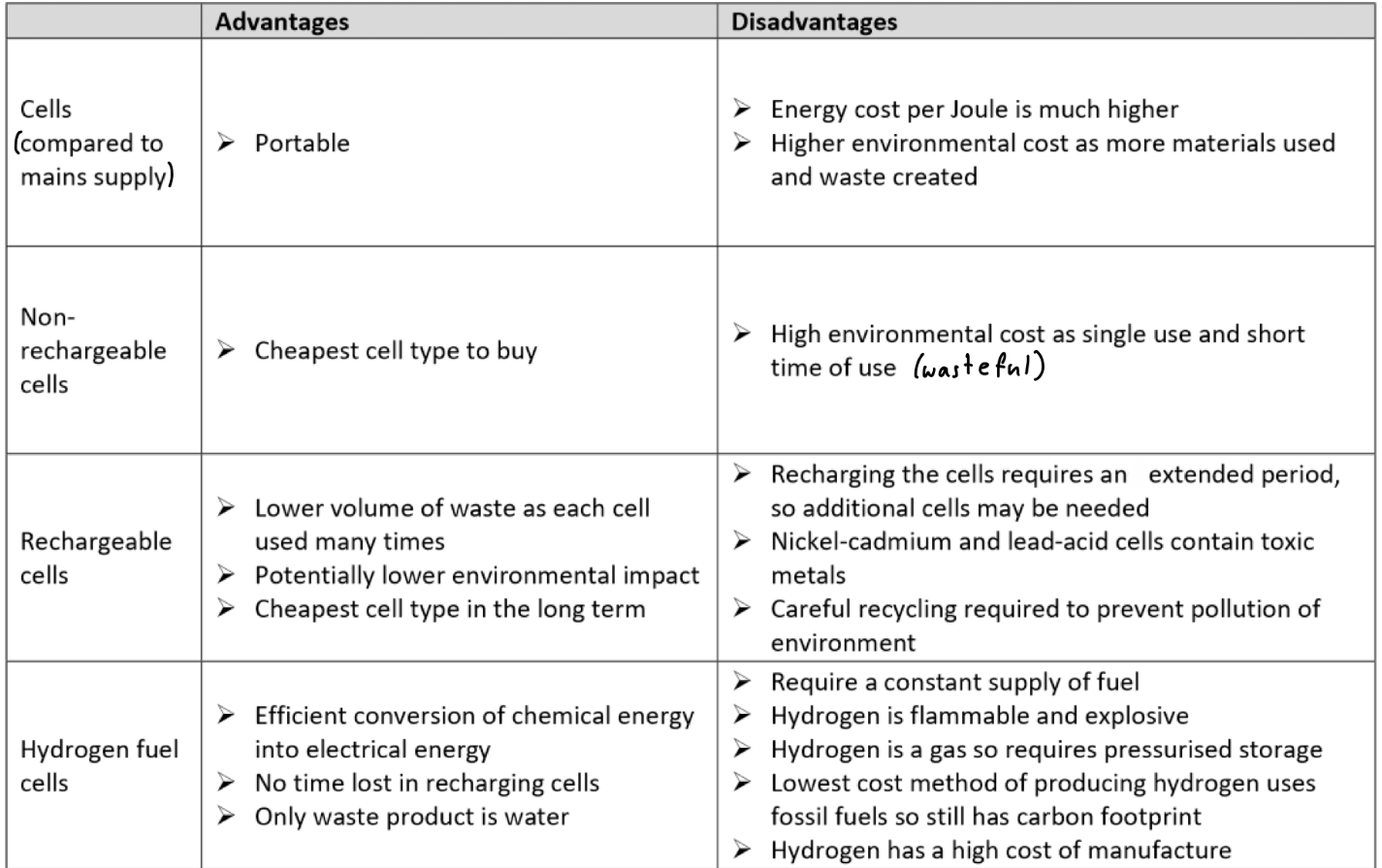
How do we represent cells?
The negative (oxidation) cell is written on the left, and the positive (reduction) cell is on the right
“||” represents a salt bridge, which separates the two sides
The electrodes on either side are written furthest from the salt bridge
Subsequent species are written with the highest oxidation number closest to the salt bridge
“|” separates species of different states
A comma separates species in the same state
If H+ ions are needed to balance the equation they are included at the end of the respective list of species
What are the standard electrode potential conditions?
Ion concentration of 1.00 mol dm-3
Temperature of 298 K
Pressure of 100 kPa
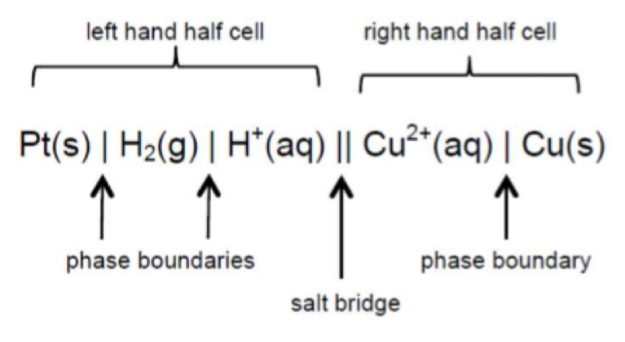
What is the standard hydrogen electrode and why is it called a reference electrode?
An inert platinum electrode in contact with both hydrogen gas and hydrogen ions in solution
When the standard hydrogen electrode is connected to another half-cell, the standard electrode potential of that half-cell can be recorded with a voltmeter
The standard electrode potential of the hydrogen electrode is taken as 0V by definition, and every other electrode potential is measured relative
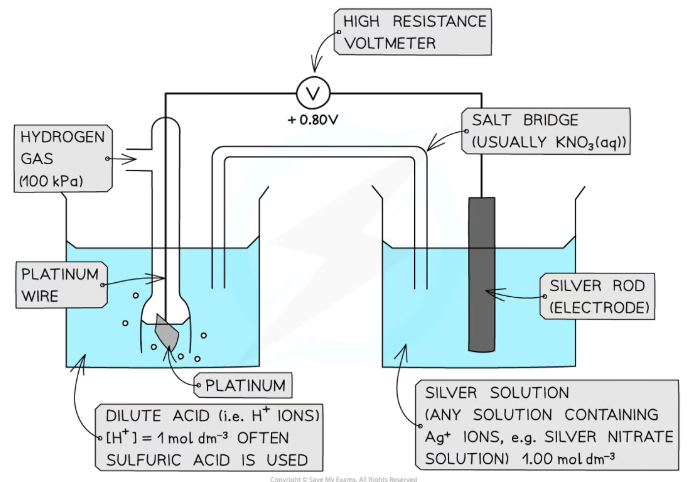
What is the EMF of a cell? How do we calculate it?
The potential difference/voltage of an electrochemical cell
This is calculated by subtracting the less positive electrode potential from the more positive one
When using the conventional representation of cells and electrode potential diagrams, this is the left side (negative electrode) subtracted from the right side (positive electrode)
EMF = Erightꝋ - Eleftꝋ
How can we predict the direction of electron flow in a cell?
By comparing the electrode potential, E, values of the two half cells:
The more negative electrode potential represents where oxidation is more likely to take place- this is the negative electrode
The more positive electrode potential represents where reduction is more likely to take place- this is the positive electrode
In the conventional representation of cells, the negative electrode is put on the left and the positive electrode on the right
What is a salt bridge and why is it needed in an electrochemical cell? What is important about the salt used?
A salt bridge connects the solutions in two half cells and completes the circuit by allowing ion movement
They are commonly soaked in a saturated solution of potassium nitrate
This is because both potassium and nitrate ions always form soluble compounds, so nothing will precipitate out of solution and affect the position of equilibrium
Why is a high-resistance voltmeter used to measure the electrode potential?
A high resistance means a low current of electrons
This increases the potential difference so that we can measure the maximum electrode potential
Describe a commercial lithium cell
The positive electrode is made of lithium cobalt oxide, (LiCoO2)
The negative electrode is made of carbon
They are separated by a solid porous polymer electrolyte
Used in laptops, phones and tablets, as they:
Are rechargeable
Are light, because lithium is the least dense metal
Don’t leak like other batteries, as the electrolyte is a solid polymer rather than a liquid or paste
Charge can be topped up without the ‘memory effect’ other batteries have, which can only be recharged efficiently once fully discharged
However, the global lithium supply is finite and running out, so these may be unsustainable- they must be recycled and the lithium saved so it isn’t wasted
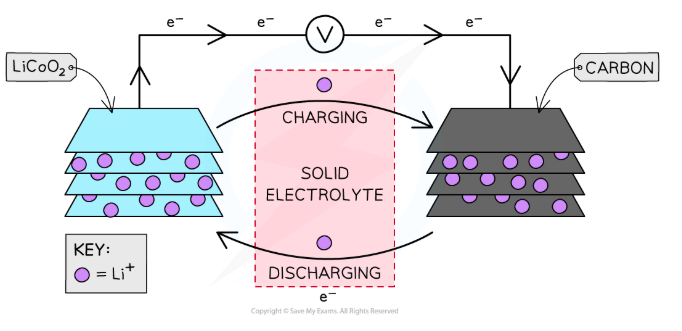
What reactions occur in a commercial lithium cell?
During discharging (when it is a galvanic cell):
At the positive electrode, reduction occurs: Eꝋ = +1.00 V
Li+ (s) + CoO2 (s) + e– → Li(CoO2) (s)
At the negative electrode, oxidation occurs: Eꝋ = -3.00 V
Li (s) → Li+ (s) + e–
So the overall reaction is: EMF = +4.00 V
Li (s) + CoO2 (s) → Li(CoO2) (s)
During charging (when it is an electrolytic cell), electrons are forced in the opposite direction through the circuit, so the reactions are reversed
What are fuel cells?
Electrochemical cells which do not run out/go ‘flat’, and do not need to be electrically recharged, as they are constantly supplied with fuel
Hydrogen-oxygen fuel cells can be used to replace petrol/diesel engines in vehicles
They just need a supply of hydrogen gas (as oxygen is obtained from the air), and only produce water
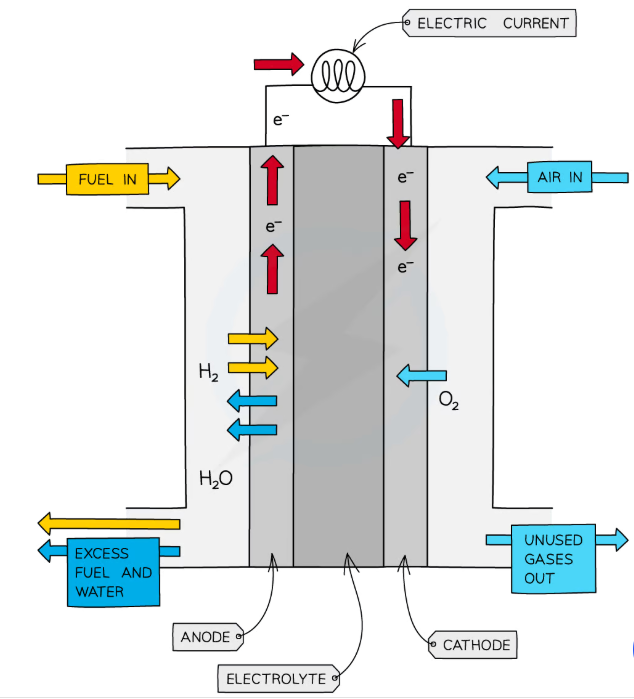
What reactions occur in an alkaline hydrogen-oxygen fuel cell?
At the positive electrode, reduction occurs: Eꝋ = +0.40 V
O2 (g) + 2H2O + 4e– → 4OH– (aq)
At the negative electrode, oxidation occurs: Eꝋ = -0.83 V
H2 (g) + 2OH– (aq) → 2H2O (l) + 2e–
So the overall reaction is: EMF = +1.23 V
2H2 (g) + O2 (g) → 2H2O (l)
What are the advantages and disadvantages of using cells compared to mains power, including non-rechargeable, rechargeable and fuel cells?