Chem- Exam 0 (Chemical Reactions, Scientific Notation, Functional Equations)
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
When balancing equations, you change _, to ensure all elements are _ on both sides of the equation.
coefficients, equal
Solubility Reaction
AB+CD —> AD+CB
Net Ionic Reaction
Aq + Aq —> S
Combution Reaction
AB+ O2 —> CO2+ H2O (always has reactant gas Oxygen and product water)
Acid Base Reaction
HB+AOH —> H2O + AB (always has H at front and OH at end of reactants, and water as a product)
Phase Change Reaction
S —> L
L —> G
etc
Displacement Reaction
AB+C —> CB+A
In reactions, oxidation number refers to the _____ in an equation.
number of oxygen atoms

What is this symbol?
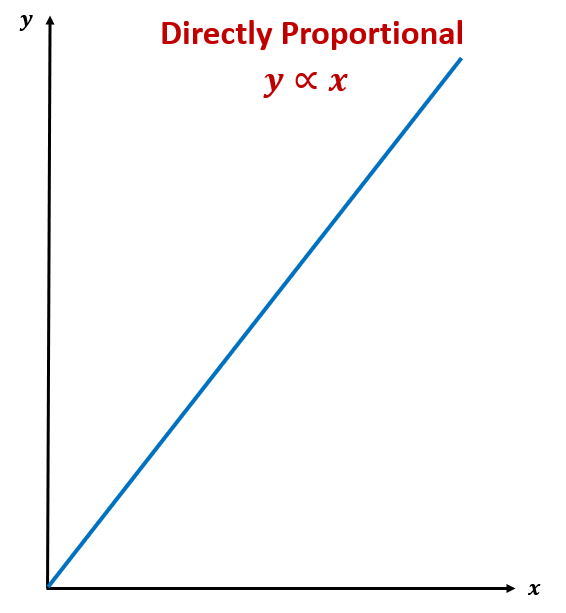
Proportionality
Directly proportional relationship
Both going up, y=X
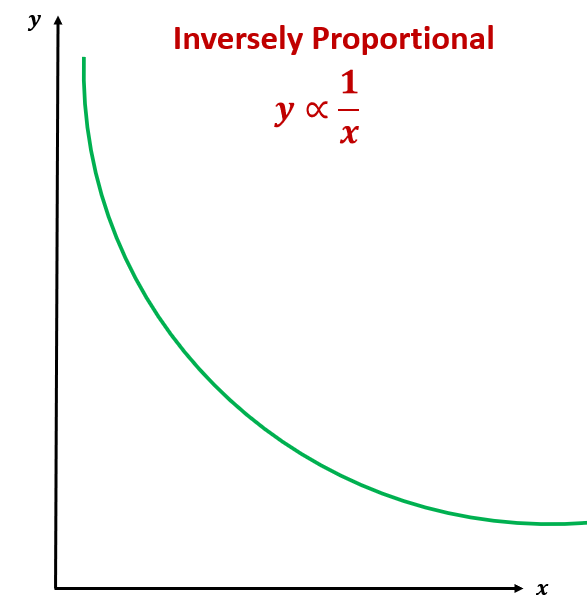
Inverse
one going up one going down, y= 1/x
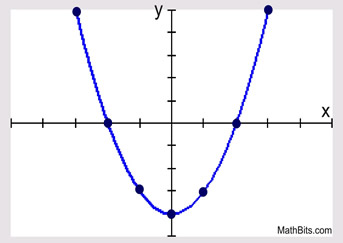
Quadratic
y= x²
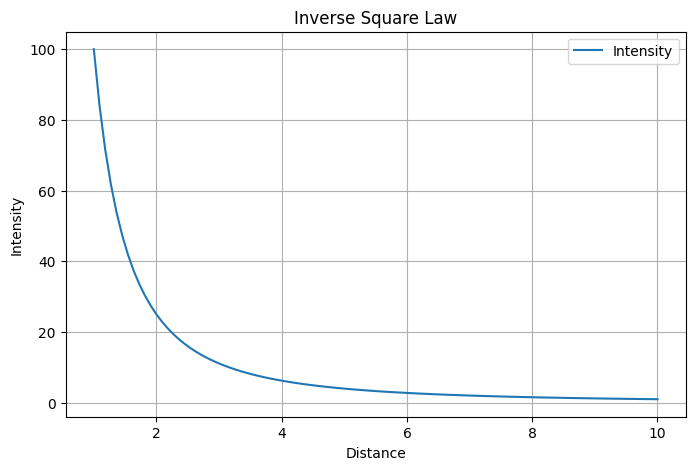
Inverse Square
y= 1/x²
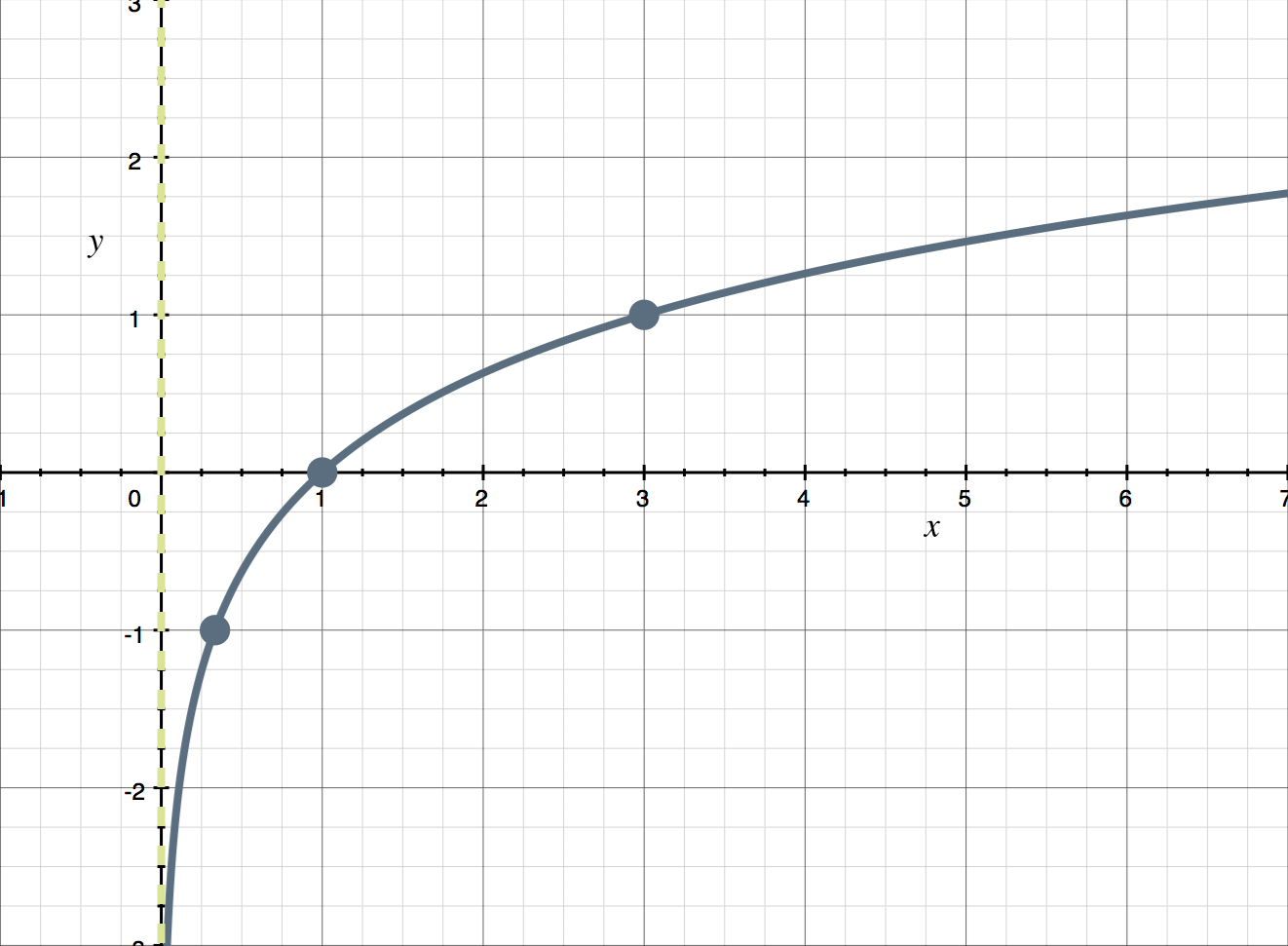
Logarithmic
y= lognx
Exponential
y= 2^x

10^9= ___ number, 10^-9=____ number
really big, really small
First numbers have to be between
1 and 9
To multiply, ___ the coefficients and ___ the exponents
multiply, add
To divide, ___ the coefficients and ___ the exponents
divide, subtract
List the SI units
meter, kilogram, second, kelvin, mole