Ch. 9 - IMF's, Liquids, Solids
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
when there are more e- (heavier)…..
LDF are stronger
Polarizability
the ability of an e- cloud to distort. Outer e- are held less tightly in larger atoms, so are more polarizable
D-D must be….
polar overall, not just polar bonds
Enthalpy of Vaporization
ΔHvap: the enthalpy Δ associated w/ vaporizing 1 mole of substance
ALWAYS ENDOTHERMIC
Condensation ΔH
ΔHcond = - ΔHvap
What causes vapor pressure?
Place a liquid in closed container: At beginning, there is net vaporization as surface molecules escape. Eventually a constant vapor pressure
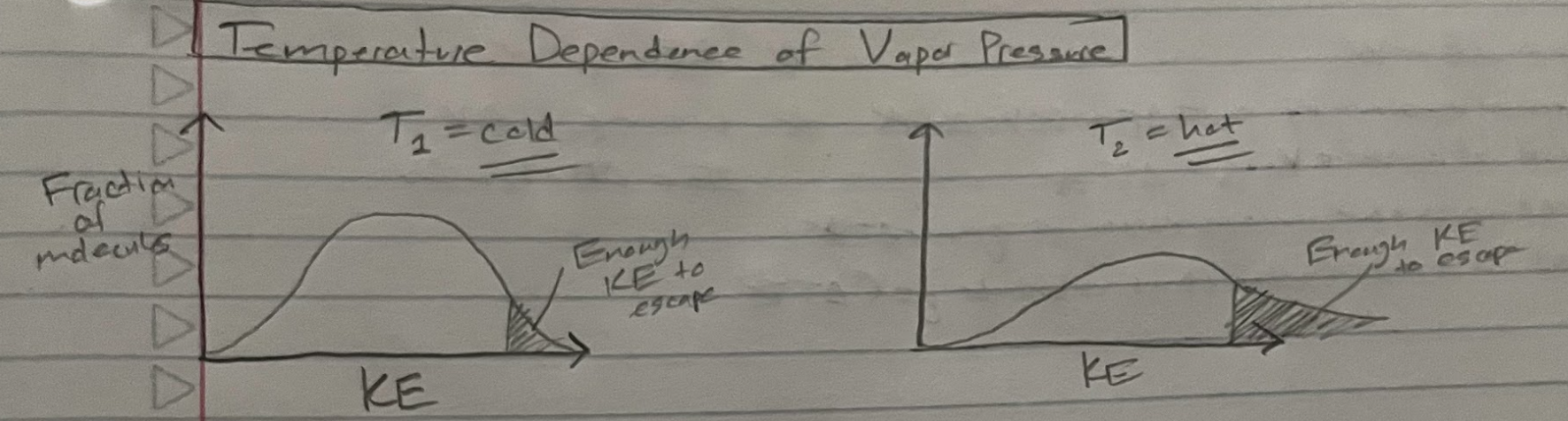
Temperature dependence of Pvap
conclusion: vap increases as T increases
Equation (Temperature dependence of Pvap)
T in Kelvin
R = 8.314 J/mol*K
when solving, at the very end you eX on both sides of eqn to get rid of ln