Quality assurance and verification/validation
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
Describe recommendations for preparation and use of reagents in the molecular laboratory.
- Document instructions for reagent preparation and volumes in the written protocol; record lot numbers and working stock preparation. Test new lots against known positive/negative samples.
- Primers commonly arrive lyophilized; resuspend to stock concentration and prepare working stocks; treat new working stocks as new reagent lots.
- Aliquot reagents (especially enzymes/proteins) to avoid repeated freeze–thaw; store according to manufacturer (many enzymes at −18 to −25°C; some reagents stable at room temp if freeze-dried). Avoid frost-free freezers for reagents (defrost cycles cause thawing). Document storage time/temperature and expiration.
- When replacing reagents, run the new lot with positive/negative controls and previously used aliquots to verify consistent performance.
What is ASR (analyte-specific reagents)
Analyte-specific reagents (ASR) = probes, primers, antibodies, or other test components that detect a specific target
- Are the active part of the laboratory developed test
- Most ASRs used in the molecular laboratory are class I, not subject to special controls by the Food and Drug Administration
- The laboratory must validate the usage of the ASR in the diagnostic test (laboratory-developed test)
- Reagents called Research Use Only (RUO) are not intended for diagnostic use and should not be used for patient testing
o May not be manufactured using Good Manufacturing Practices (GMP)
o Values, stability, or other quality characteristics may not be controlled
- ASR (Analyte-Specific Reagent): probes/primers/antibodies/etc. that detect a specific analyte. ASRs are classed I–III; most molecular-lab ASRs are class I and their performance is established during validation of laboratory-developed tests (LDTs).
What is research use only (RUO)
Research Use Only (RUO) =
- Not intended for diagnostic use and should not be used for patient testing
o May not be manufactured using Good Manufacturing Practices (GMP)
Values, stability, or other quality characteristics may not be controlled
- RUO (Research Use Only) / IUO (Investigational Use Only): RUO reagents are not intended for patient diagnostics; IUO reagents may be used on patient samples only under controlled research/consent (clinical trials). These are not for routine diagnostic use.
What is IVD
- IVD (In-Vitro Diagnostic device/reagent set): intended for diagnostic use on patient specimens; IVDs have risk-based classes (I–III); laboratories must verify performance (accuracy, precision, AMR, etc.) when they implement an IVD and must validate if they modify it.
Know the pic
the pic
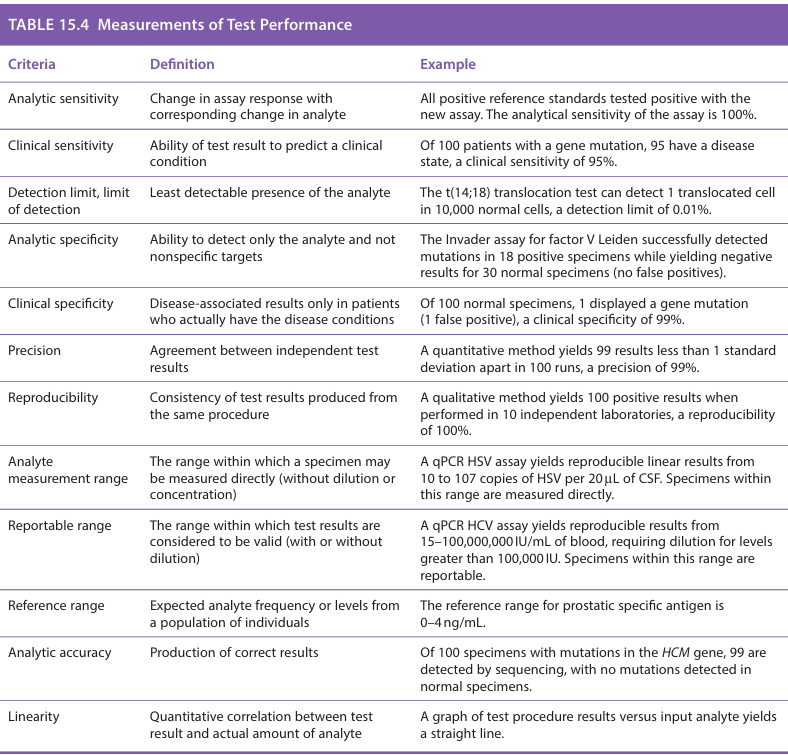
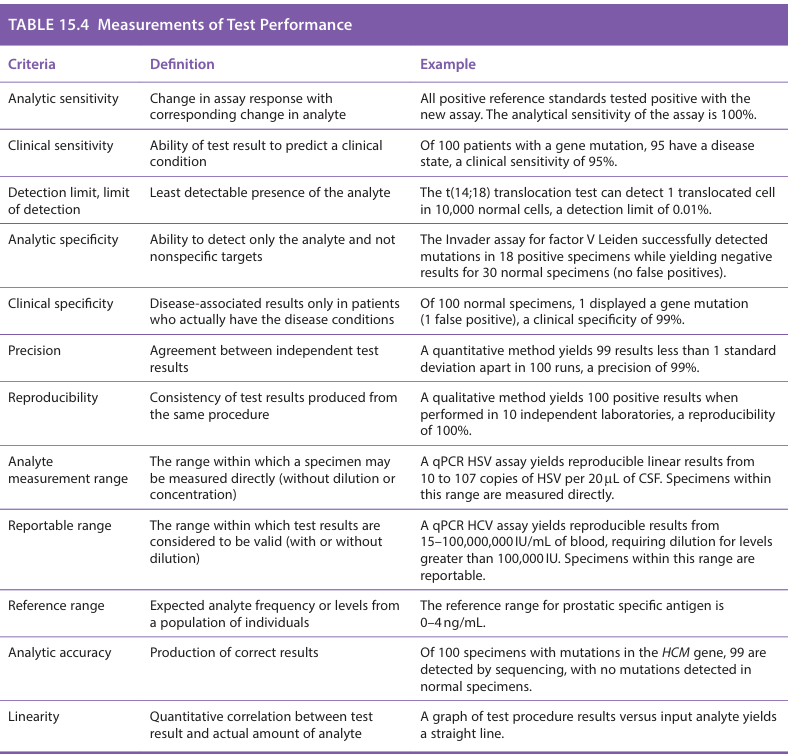
What is analytic sensitivity
Analytic sensitivity: change in assay response with corresponding change in analyte
ex: All positive reference standards tested positive with the new assay. The analytical sensitivity of the assay is 100%
What is clinical sensitivity
Ability of the test result to predict a clinical condition.
Ex: Of 100 patients with a gene mutation, 95 have a disease state, a clinical sensitivity of 95%
What is detection limit, limit of detection?
lowest reliably detected analyte level. (least detectable presence of the analyte)
Ex: The t(14;18) translocation test can detect 1 translocated cell in 10,000 normal cells, a detection limit of 0.01%
What is analytic specificity
The ability to detect only the analyte and not nonspecific targets
Ex: The invader assay for factor V Leiden successfully detected mutations in 18 positive specimens while yielding negative results for 30 normal specimens (no false positives)
What is Clinical specificity
Disease-associated results only in patients who actually have the disease conditions
Ex: Of 100 normal specimens, 1 displayed a gene mutation (1 false positive), a clinical specificity of 99%
What is precision
Agreement between independent test results
Ex: A quantitative method yields 99 results less than 1 standard deviation apart in 100 runs, a precision of 99%
What is reproducibility
Consistency of test results produced from the same procedure
Ex: A qualitative method yields 100 positive results when performed in 10 independent laboratories, a reproducibility of 100%
What is analyte measurement range
The range within which a specimen may be measured directly (without dilution or concentration)
Ex: A qPCR HSV assay yields reproducible linear results from 10 to 107 copies of HSV per 20 uL of CSF. Specimens within this range are measured directly
What is reportable range
The range within which test results are considered to be valid (with or without dilution)
Ex: A qPCR HCV assay yields reproducible results from 15-100,000 IU/mL of blood, requiring dilution for levels greater than 100,000 IU. Specimens within this range are reportable
What is reference range
The expected analyte frequency or levels from a population of individuals
Ex: The reference range for prostatic specific antigen is 0-4 ng/mL
What is analytic accuracy
Production of correct results
EX: Of 100 specimens with mutations in the HCM gene, 99 are detected by sequencing, with no mutations detected in normal specimens
What is linearity
Quantitative correlation between test result and actual amount of analyte
Ex: A graph of test procedure results versus input analyte yields a straight line
What is verification
Verification = verifying test performance criteria according to the package insert for an FDA-approved test (“in vitro diagnostic”)
- FDA-approved (minimum requirements)
o Clinical sensitivity
o Clinical specificity
o Accuracy
o Precision
- Verification: for FDA-approved/cleared tests the lab verifies that the assay performs in the local lab as expected (accuracy, precision, reportable range, reference range) when run according to manufacturer instructions. If unmodified, only verification is required.
What is validation
Validation = establishing test performance criteria in a Lab-Developed Test (LDT, aka “home brew”)
- Minimum requirement (LDT) - more shown on table
o Clinical sensitivity
o Analytical sensitivity/limit of detection
o Clinical specificity
o Analytical specificity
o Accuracy
o Precision
o Reportable range/analytical measurement range (what can I report – goes to the doctor)
§ Ex: genotypes, standard curve values
o Reference range (the value you expect in a health individual)
o Linearity
- Validation: required when the lab develops its own test (LDT) or modifies a commercial assay. Validation is the broader process demonstrating the procedure is ready for clinical use — includes establishing sensitivity, specificity, AMR, precision, reproducibility, detection limits, and clinical performance using appropriate specimen types. Validation documents clinical utility and test parameters.
What is test performance validation
- Federal regulations from CLIA require validation of the performance of clinical test methods and reagents in accurately detecting or measuring analytes prior to use in human testing
- Test validation is performed on specimens of types that will be encountered in the routine use of the test
- The number of specimens tested varies with the procedure and availability of test material
- Results from the new test methodology are compared to results from established procedures or correlated to clinical diagnosis
What is clinical sensitivity
Clinical sensitivity = relates to false negative rate
DIFFER FROM ANALYTICAL SENSITIVITY AND SPECIFICITY
From pic…
TP = true positive, TN = true negative,
FN = false negative, FP = false positive

What is clinical specificity
Clinical specificity = relates to false positive rate
DIFFER FROM ANALYTICAL SENSITIVITY AND SPECIFICITY
From pic…
TP = true positive, TN = true negative,
FN = false negative, FP = false positive

What is positive predictive value (PPV)
Positive Predictive Value (PPV) = the likelihood of disease given a positive result
- Highly dependent on the prevalence of the disease
- Increases with higher prevalence, which means rare diseases have poor PPVs, even with good sensitivity and specificity
the degree that a particular test result corresponds with the presence of a clinical state”

What is proficiency testing
Proficiency testing (ask about during rotations)
- External specimens supplied by a third-party from a known reference source
- Assesses the competency of the laboratory, blind-samples
o Tested at least twice per year, in routine patient runs
- Supplied by CAP
- If not available, labs must do split-sample testing
o Partnership between two labs, blind each other to specimen results, compare results after
- Proficiency testing results may NEVER be shared between labs and PT samples can NEVER be sent-out to another lab for testing (no PT referral)
The provided reading references proficiency testing material (e.g., as potential calibrators) and notes proficiency testing influences calibration/recalibration decisions, but it does not give a step-by-step description of the purpose/process of proficiency testing in the excerpted pages. Therefore, I’m skipping an expanded description per your instruction (the reading did not include sufficient detail).
How do labs handle safety with chemicals and other factors?
Safety
- Every lab must have safety procedures established and routinely followed
- Includes biohazardous safety, fire safety, electrical safety, and chemical safety
- Some labs still use radioactivity, so special monitoring is performed (exposure badges)
- Every chemical and waste container must be properly labeled and stored
Hazardous chemicals: Transport and Storage
- Secondary or reinforced containers are required for transport and handling of dangerous chemicals such as concentrated acids or phenol
- Volatile and flammable reagents are stored in properly vented and explosion-proof cabinets or refrigeration units
Identify and give examples of safety requirements in the laboratory.
· Chemical safety: store volatile/flammable reagents (xylene, methanol, ethanol, isopropanol) in vented/explosion-proof cabinets or refrigerators; use secondary containers for concentrated acids/phenol.
· Radiation safety: radioactive reagents handled in designated areas; radiation safety manual required; workspaces covered/protected; radiation badges for handling ≥1.0 mCi; acrylic shielding for isotopes like ³²P; waste disposal per regulations; periodic contamination monitoring (swipe tests, Geiger).
· PPE & engineering controls: gloves, lab coat, safety glasses; fume hoods and laminar-flow hoods monitored and certified; refrigerators/freezers and incubators monitored for temperatures; instruments maintained per manufacturer.
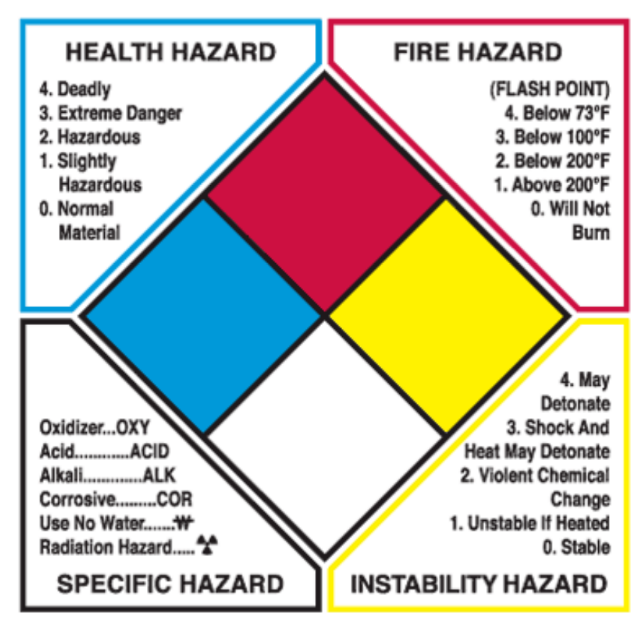
What are the hazards on the NFPA hazard diamond.
Hazardous chemicals
- Developed by the National Fire Protection Association, warning labels for universal use on all chemical containers
The NFPA diamond is three numbered sections plus a specific-hazard field:
· Health (left): 0 (none) → 4 (deadly).
· Flammability / Fire hazard (top): 0 (will not burn) → 4 (below 73°F flash point = extreme).
· Reactivity (right): 0 (stable) → 4 (may detonate/deteriorate).
· Specific hazard (bottom): OXY (oxidizer), ACID, ALK, COR (corrosive), W (use no water), radiation symbol, etc. The figure in the reading explains the numeric scale and specific-hazard codes