Chapter 7: Periodic Table, Ionisation Energies, and Bonding + Structure
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
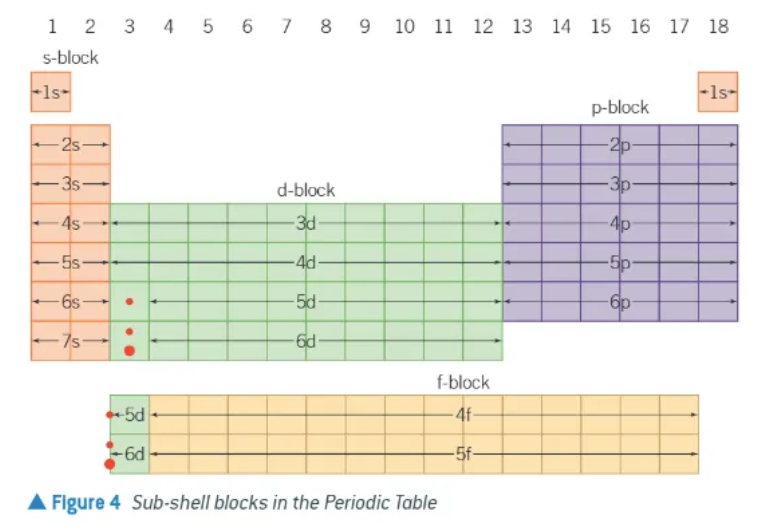
where are the spdf blocks??
first ionisation energy definition:
the energy required to remove one electron from each atom in one mole of gaseous atoms of an element to form one mole of gaseous +1 ions
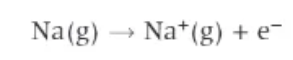
what 3 factors affect an ionisation energy?
atomic radius
nuclear charge
electron shielding
the first electron to be removed from an atom will be in the…
highest energy level → experiencing the least attraction from the nucleus
how does atomic radius affect ionisation energy?
the larger the atomic radius, the weaker the attraction between the nucleus + outer electrons. This means it is easier to lose an outer electron
how does nuclear charge affect ionisation energy?
more protons → greater attraction between nucleus + outer electrons
how does electron shielding affect ionisation energy?
electrons = negatively charged
inner shells repel outer shells
this repulsion = ‘shielding effect’
easier for an outer electron to be lost
write the first 2 ionisation energies for helium:

why do successive ionisation energies increase? (in general!)
after the first electron is lost, the remaining electron/s are pulled closer to the nucleus
this remaining nuclear attraction increases
more ionisation energy = required to remove this second electron
def. of second ionisation energy:
energy required to remove one electron from each ion in one mole of gaseous +1 ions of an element to form one mole of gaseous +2 ions.
what charged ion does a 2nd ionisation energy produce?
+2 ion
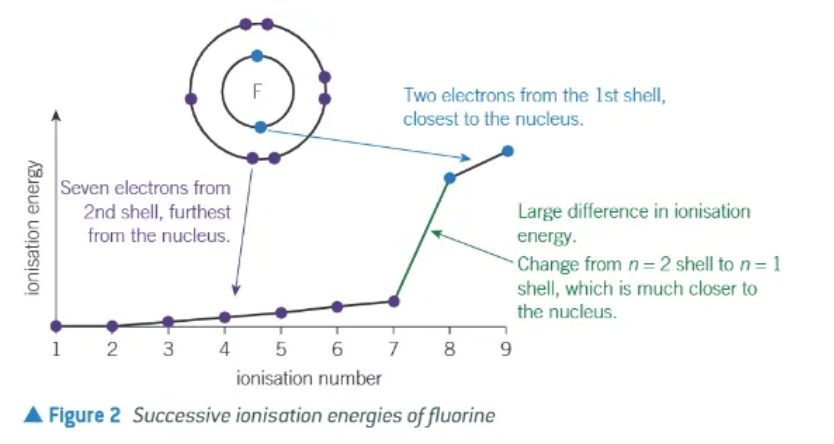
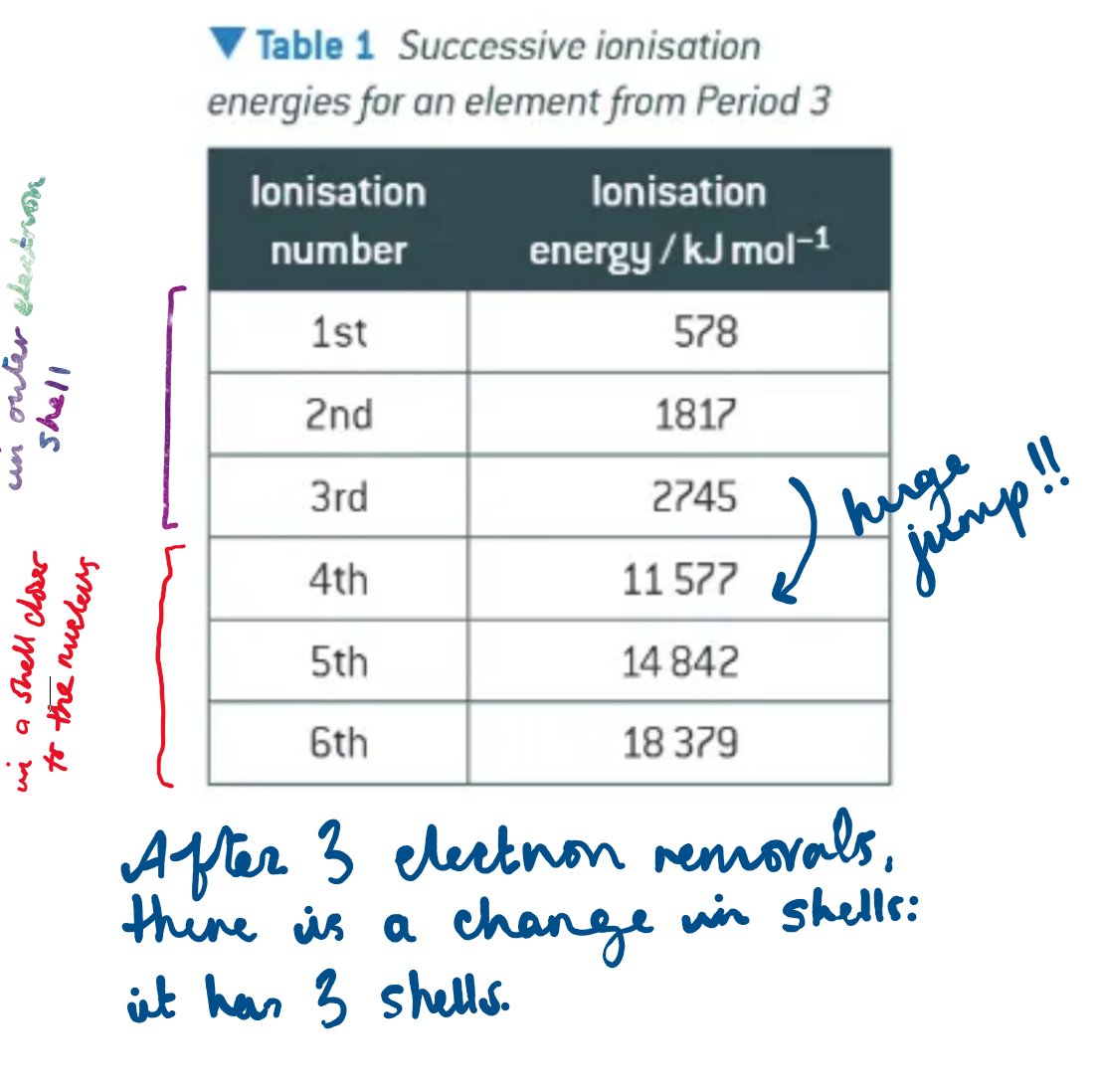
what does a big jump upwards between successive ionisation energies tell us?
that the higher numbered electron is removed from a different electron shell, closer to the nucleus + with less shielding
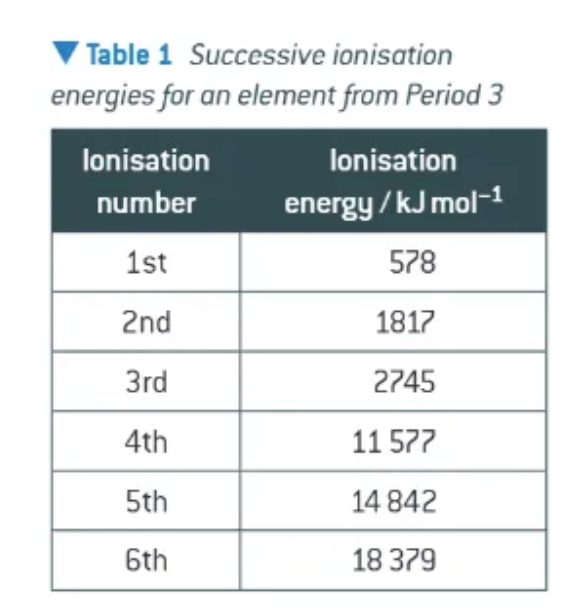
what does this table tell us?
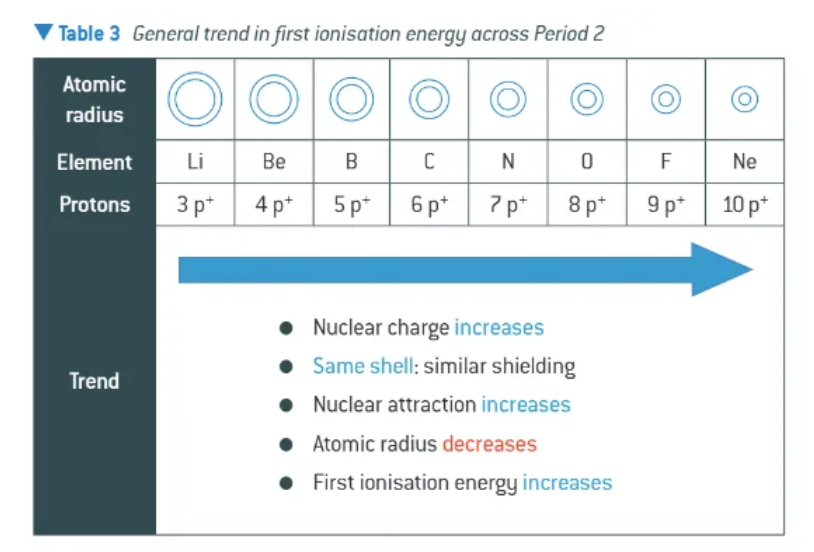
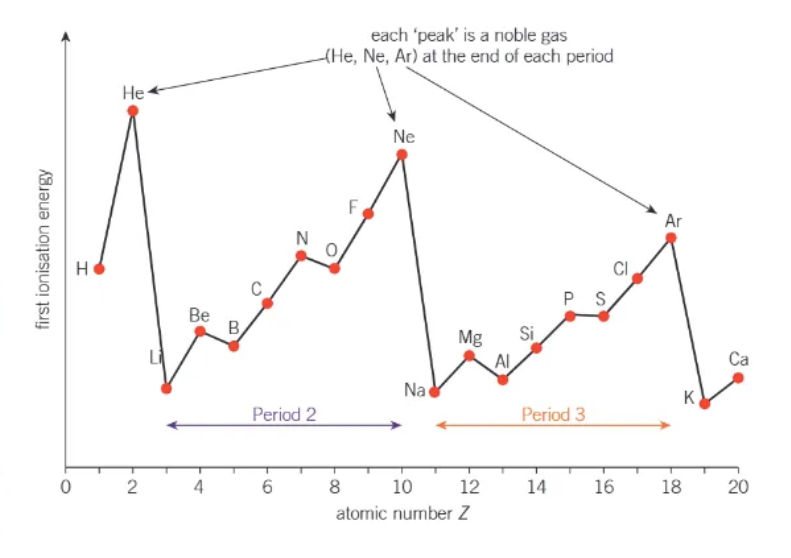
how do 1st ionisation energies changes going across the period (→) GENERALLY SPEAKING !!
general increase across each period 📈📈
sharp decrease in first ionisation energy between the end of one period + the start of the next
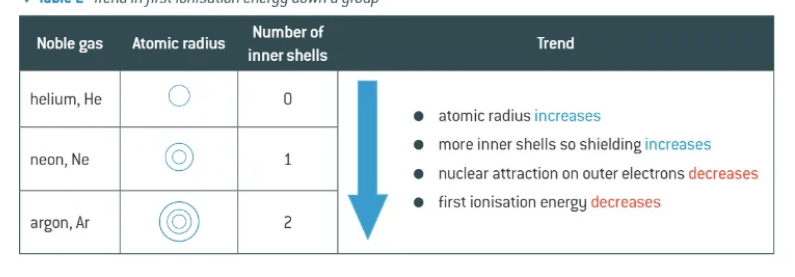
trend in first ionisation energy down a group:
decrease down a group
look at He, Ne, Ar
look at Li, Na, K
why do 1st ionisation energies decrease down a group?
what is the most important factor as to why first ionisation energies increase across a period?
increase nuclear charge
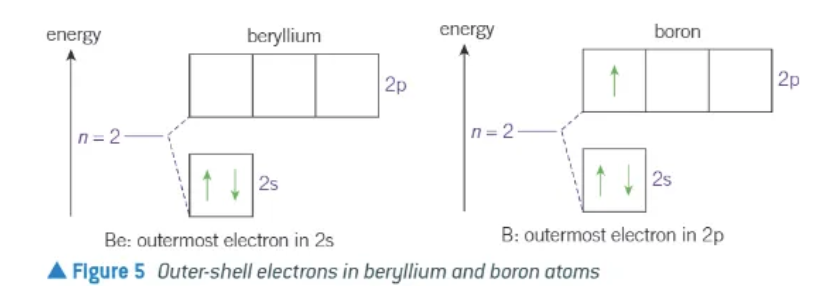
in periods 2 + 3, why is there not a complete increase in 1st ionisation energies across the period (Beryllium → Boro)
Beryllium + Boron
marks the start of the filling of the 2p subshell
2p subshell has a higher energy subshell
therefore, easier to remove than one of the 2s electrons in beryllium
first ionisation energy of boron is less than the first i.e. of beryllium
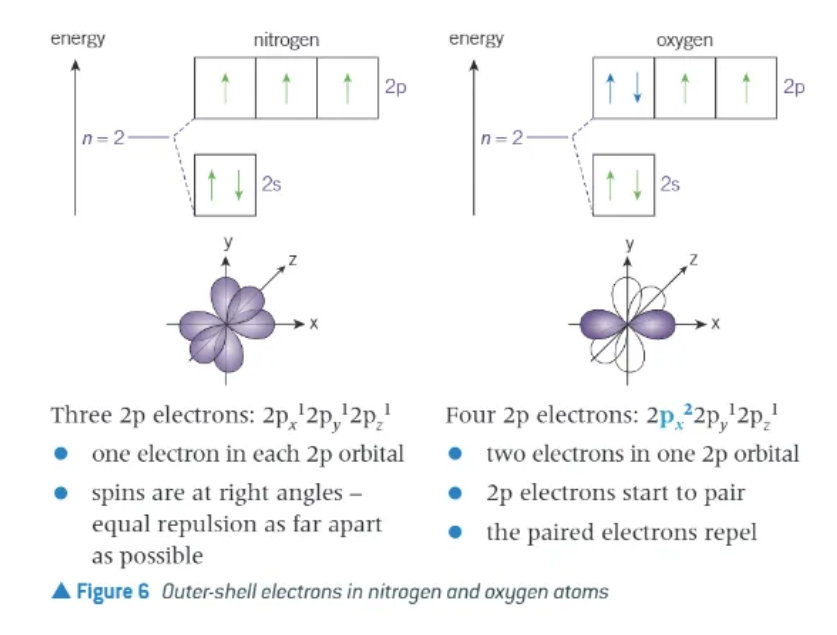
in periods 2 + 3, why is there a decrease in successive ionisation energies between N → O
marks the first electron pair to be in an orbital (2p orbital)
the paired electrons repel, making it easier to remove and electron from an oxygen atom than a nitrogen atom
first ionisation energy of oxygen is less than the first ionisation energy of nitrogen
metallic bonding:
cations fixed in positions, and delocalised electrons are free to move + charry a charge
for metals containing +2 cations, how many electrons are needed?
twice as many as the cations, to balance the charge
what kind of structure does a metal have?
giant metallic lattice
3 properties of metals:
strong metallic bonds
high electrical conductivity
high melting + boiling points
why do metals have a high boiling point?
large amounts of energy are required to overcome the strong electrostatic attraction between the cations + electrons
why are most metals insoluble?
you might expect polar solvents to interact with charges in metallic lattice
this doesn’t happen → reactions occur instead!!
simple molecular lattices are held together by…
weak intermolecular forces
carbon + silicon form…
giant covalent lattices:
tetrahedral
high melting + boiling points → strong covalent bond
insoluble → strong covalent bonds cannot be broken by interactions with solvents
non-conductors of electricity (mostly!) → no electrons free to move and carry a charge
which two allotropes of carbon can conduct electricity?
graphene + graphite
only 3/4 of the outer electrons are used in covalent bonds
remaining electron is in a pool of delocalised electrons + are free to move
graphite is…
a stack of graphene layers (with weak forces between the layers)
do giant structures have high mp? do simple structures?
giant = yes → have strong forces to overcome
simple = no → have weak forces to overcome
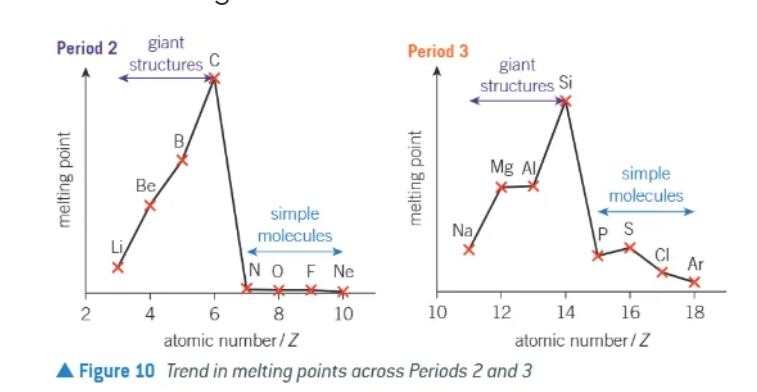
melting points across a period
increases between groups 1 → 4
sharp decrease between group 4 → 5
melting points are comparatively low from groups 5 → 8
which 3 elements form giant covalent structures?
Boron, Carbon, and Silicon
what do Phosphorus and Sulphur exist as?
P4 + S8