A level Chemistry - Atomic Structure
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
Explain how the understanding of atomic structure has evolved over time
Robert Boyle: proposed there was some substance that could not be made simpler
John Dalton: all elements are made of atom, atoms of the same element have the same mass, elements of different elements have different mass
Henri Becquerel: discovered radioactive, proved atoms have internal energy and can change
J.J Thompson: cathode ray tube experiment. proposed plum pudding model, atoms are a sphere of positive charge with negatively charged electrons embedded within it
Earnest Rutherford: alpha particle scattering experiment. Rutherford fired tiny, positively charged particles at a very thin sheet of gold foil.
What happened | What he concluded | |
most particles went straight through | atoms are mostly empty space | |
Some particles deflected a little | Tiny |
Explain how the understanding of atomic structure has evolved over time
Robert Boyle: proposed there was some substance that could not be made simpler
John Dalton: all elements are made of atom, atoms of the same element have the same mass, elements of different elements have different mass
Henri Becquerel: discovered radioactive, proved atoms have internal energy and can change
J.J Thompson: cathode ray tube experiment. proposed plum pudding model, atoms are a sphere of positive charge with negatively charged electrons embedded within it
Earnest Rutherford: alpha particle scattering experiment. Rutherford fired tiny, positively charged particles at a very thin sheet of gold foil.
What happened:
- Most particles went straight through —> concluded that atoms are mostly empty space
-Some particles deflected a little , a few bounced straight back—> concluded that there is a tiny, dense positively charged nucleus at the centre of the atom
-he concluded that electrons move around the outside of the nucleus
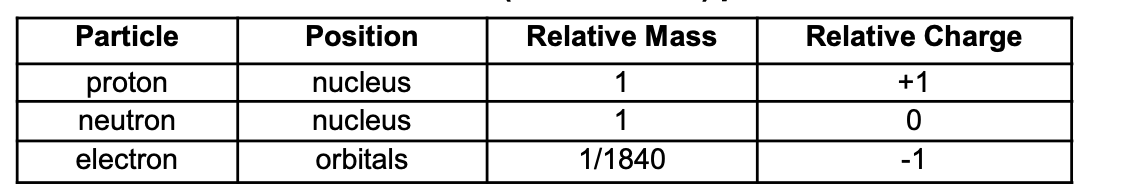
State the relative mass and charge of a proton, electron and neutron
Define the term isotope
Isotopes are atoms with the same number of protons, but different numbers of neutrons.
Why might isotopes have similar chemical properties but vary in physical properties
Isotopes have similar chemical properties because they have the same electronic structure. They may have slightly varying physical properties because they have different masses.
How do you calculate relative formula mass?
What can a mass spectrometer be used for?
The mass spectrometer can be used to determine all the isotopes present in a sample of an element and to therefore identify elements.
What are the main steps of Time Of Flight mass spectrometry?
Vaporisation : The sample is turned into a gas so that particles are separated and can be ionised effectively. This is often done by heating the sample.
Ionisation
Acceleration
Drift / Flight tube
Detection
What are the two main techniques of ionisation?
Electron Impact
Electro -Spray
How is electron impact carried out
1) Electron impact:
•A vaporised sample is injected at low pressure into the electron chamber
•An electron gun fires high energy electrons at the sample
•This knocks out an outer electron
•Forming positive ions with different charges e.g. Ti (g) —> Ti+ (g)+ e-
Key info:
- low pressure —> so that ionisation is more controlled, as molecules do not collide with each other
-Electron impact is used for elements and substances with low formula mass. Electron impact can cause larger organic molecules to fragment.
What is fragmentation?
when the molecule breaks into smaller charges pieces
After ionisation, the molecular ion (M⁺) often has excess energy.
This causes bonds to break, splitting the molecule into smaller ions.
These smaller ions are called fragments.
Fragmentation creates multiple peaks at lower m/z values.
Downside: Can make the molecular ion peak (M⁺) weak or hard to spot → harder to find the molecular mass.
Useful: Fragment pattern acts as a fingerprint to help identify the molecule’s structure.
How does electro-spray ionisation work?
Electro spray Ionisation
• The sample is dissolved in a volatile, polar solvent
• injected through a fine needle giving a fine mist or aerosol
• the tip of needle has high voltage
• at the tip of the needle the sample molecule, M, gains a proton, H+, from the
solvent forming MH+
• M(g) + H+ —> MH+(g)
• The solvent evaporates away while the MH+ ions move towards a negative plate
Electro spray ionisation is used preferably for larger organic molecules. The ‘softer’ conditions of this technique mean fragmentation does not occur.
What occurs during acceleration?
•Positive ions are accelerated by an electric field
•To a constant kinetic energy
What occurs during drift / flight tube?
•The positive ions with smaller m/z values will have the same kinetic energy as those with larger m/z values, so will move faster
•The heavier particles take longer to move through the drift area.
•The ions are distinguished by different flight times
What occurs during detection?
The ions reach the detector and generate a small current, which is fed to a computer for analysis.
The current is produced by electrons transferring from the detector to the positive ions.
The size of the current is proportional to the abundance of the species
what values can the mass spectrometer for each isotope?
m/z ( mass/charge) ratio
relative abundance
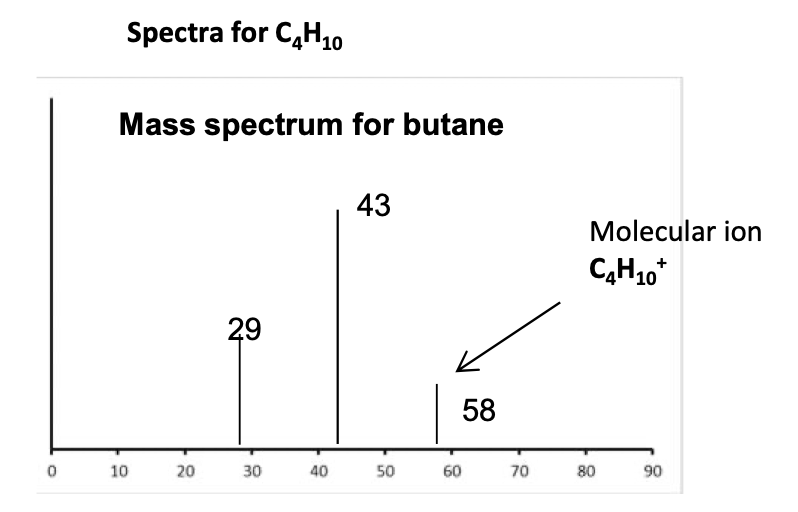
How do you measure the Mr of a molecule using the TOF spectra?
If a molecule is put through a mass spectrometer with an Electron impact ionisation stage it will often break up and give a series of peaks caused by the fragments. The peak with the largest m/z, however, will be due to the complete molecule and will be equal to the relative molecular mass , Mr ,of the molecule. This peak is called the parent ion or molecular ion
If a molecule is put through a mass spectrometer with electro spray ionisation then fragmentation will not occur. There will be one peak that will equal the mass of the MH+ ion. It will therefore be necessary to subtract 1 to get the Mr of the molecule. So if a peak at 521.1 is for MH+, the relative molecular mass of the molecule is 520.1.
How are electrons arranged?
1)Principle energy levels numbered 1,2,3,4..
1 is closest to nucleus
2) Split into: Sub energy levels labeled s, p , d , f.
s holds up to 2 electrons
p holds up to 6 electrons
d holds up to 10 electrons
f holds up to 14 electrons
3) split into orbitals, which hold up to 2 electrons with opposite spin
What is the normal filling order of sub shells? name the 2 exceptions
An atom fills up the sub shells in order of increasing energy (note 3d is higher in energy than 4s and so gets filled after the 4s)
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p…
exceptions are chromium and copper*******
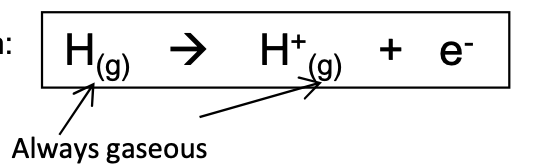
What is the definition and equation of the first ionisation energy?
the minimum energy required to remove one mole of electrons from one mole of atoms in the gaseous state to form one mole of gaseous ions with a single positive charge.
What is the definition and equation of the second ionisation energy
The second ionisation energy (IE2) is the energy required to remove the second mole of electrons from each +1 ion in a mole of gaseous +1 ions, to form one mole of +2 ions

What are the three main factors which affect ionisation energy?
1.The attraction of the nucleus
(The more protons in the nucleus the greater the attraction)
2. The distance of the electrons from the nucleus
(The bigger the atom the further the outer electrons are from the nucleus and the
weaker the attraction to the nucleus)
3. Shielding of the attraction of the nucleus
(An electron in an outer shell is repelled by electrons in complete inner shells, weakening the attraction of the nucleus)
why are successive ionisation energies always higher?
The second ionisation energy of an element is always bigger than the first ionisation energy.
When the first electron is removed, a positive ion is formed.
The ion increases the attraction on the remaining electrons ( as there is a greater nuclear charge) and so the energy required to remove the next electron is larger.
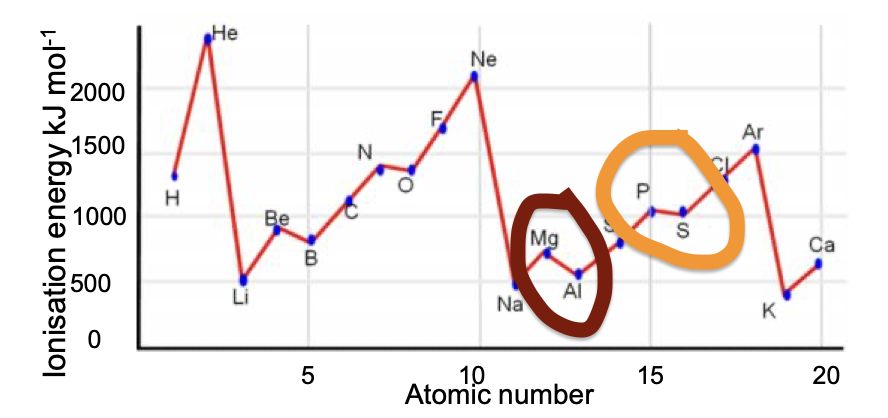
. Why has helium the largest first ionisation energy?
Why has helium the largest first ionisation energy?
Its first electron is in the first shell closest to the nucleus and has no shielding effects from inner shells. He has a bigger first ionisation energy than H as it has one more proton
Why do first ionisation energies decrease down a group?
As one goes down a group, the outer electrons are found in shells further from the nucleus and are more shielded so the attraction of the nucleus becomes smaller
Why is there a general increase in first ionisation energy across a period?
As one goes across a period the electrons are being added to the same shell which has the same distance from the nucleus and same shielding effect. The number of protons increases, however, making the effective attraction of the nucleus greater. (. higher nuclear charge)
Why has Na a much lower first ionisation energy than neon?
This is because Na will have its outer electron in a 3s shell further from the nucleus and is more shielded. So Na’s outer electron is easier to remove and has a lower ionisation energy.
Why is there a small drop from Mg to Al?
Al is starting to fill a 3p sub shell, whereas Mg has its outer electrons in the 3s sub shell.
The electrons in the 3p subshell are slightly easier to remove because the 3p electrons are higher in energy and are also slightly shielded by the 3s electrons
Why is there a small drop from P to S?
With sulfur there are 4 electrons in the 3p sub shell and the 4th is starting to doubly fill the first 3p orbital.
When the second electron is added to a 3p orbital there is a slight repulsion between the two negatively charged electrons which makes the second electron easier to remove.