NMR and IR spectroscopy
1/58
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
59 Terms
What is NMR?
-Some nuclei have a quantum mechanical property called ‘spin’ • This includes 1H, 13C, 15N, 19F, 31P nuclei, which are therefore NMR visible but NOT nuclei of the common 12C, 14N, 16O isotopes – this means they will be NMR invisible
-Spin gives nuclei magnetic properties – you can imagine a little magnet attached to each nucleus
What is the Zeeman effect?
-spin is split into energy levels
-Ιn the presence of a magnetic field, the spins split into two energy levels:
low energy, ‘spin up’, aligned WITH the field
high energy, ‘spin down’, aligned AGAINST the field
-it’s proportional to the magnetic field
-This phenomenon is called the Zeeman effect, and it’s the fundamental basis for NMR a
How does the Zeeman effect link to NMR?
-Transitions between energy levels – when spins flip between up and down – gives rise to NMR signals.
In the Zeeman effect, what does the frequency of a transition between 1 energy level to the other depend on? (2)
-the strength of the magnetic field at the nucleus
-the type of nucleus, determined by a constant called the gyromagnetic ratio
What is the origin of a ‘chemical shift’? How does this relate to NMR?
-Electrons moving around the nucleus create opposing magnetic fields that shield the nucleus from the external magnetic field This results in small frequency changes
-electron shielding changes the energy difference and NMR frequency
Are all nuclei NMR active i.e. have magnetic properties suitable for NMR spectroscopy?
-no
Give examples of atoms that are highly NMR-active
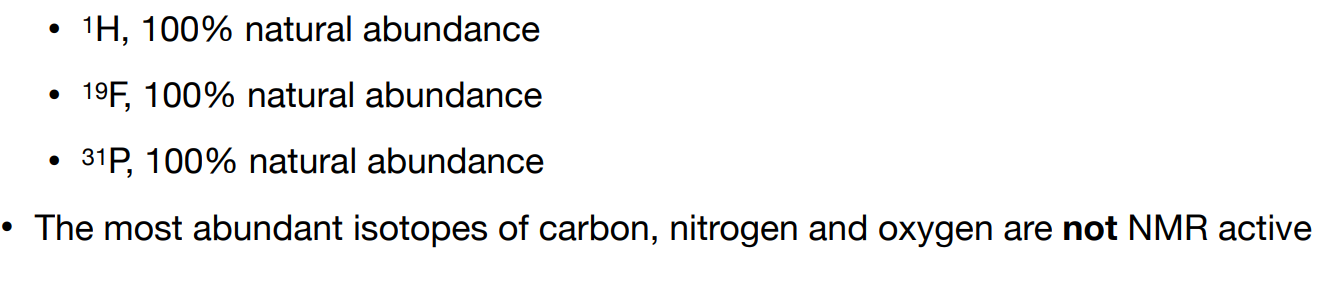
The rare isotope 13C is NMR-active. What does this mean about its sensitivity?
-13C NMR is low sensitivity
-1.1% natural abundance. The lower the natural abundance, the lower the sensitivity
How does NMR work practically?
-Samples are dissolved in a deuterated solvent, usually CDCl3 (deuterochloroform), and placed in a strong magnetic field. A radiofrequency pulse, generated by the console, is then applied to the sample, perturbing the nuclear spin of magnetically-active nuclei like 1H and 13C. The energies associated with these transitions are recorded by the detector to generate a spectrum for analysis
Why do we use a deuterated solvent for NMR?
-because it’s NMR invisible
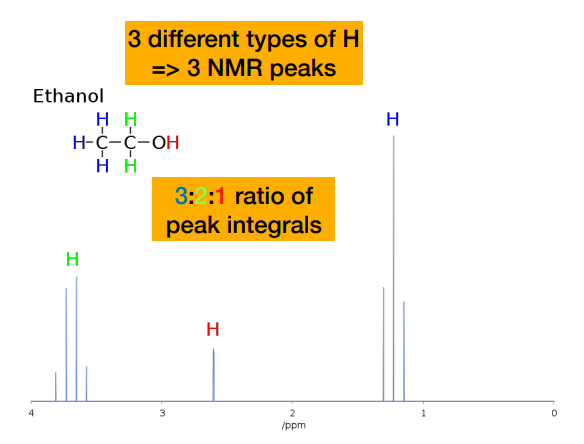
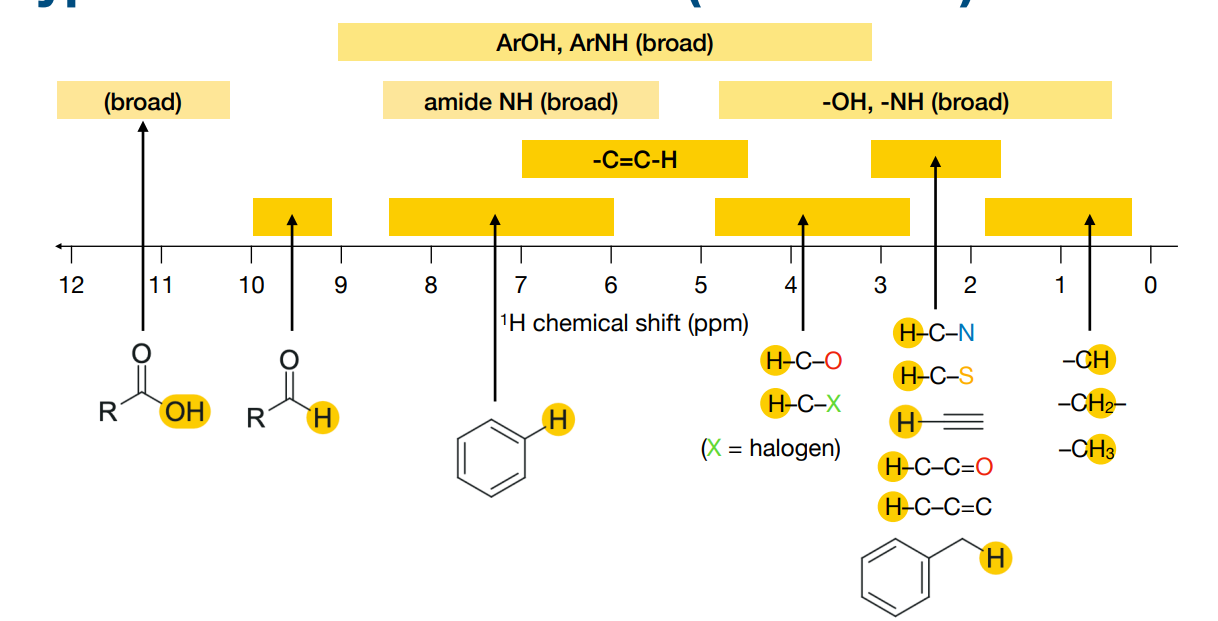
What information is in a proton (1H) NMR spectrum ?
-Number of peaks:
• the number of distinct 1H chemical environments
• related to molecular symmetry
-Area of peaks:
• called the ‘integral’ or ‘peak integration’
• this tells you how many 1H are found in this chemical environment
-Position of peaks:
• the chemical shift – this has information on the type of environment
• depends on nearby functional groups
-Shape of peaks:
• called ‘multiplicity’, ‘scalar coupling’ or ‘J coupling’
• this tells you about other 1H nearby
• e.g. the 3 lines on the left is called a triplet
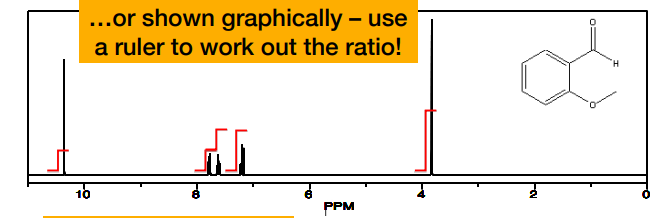
What is this?
-integrals i.e. areas for nearby peaks are sometimes joined together
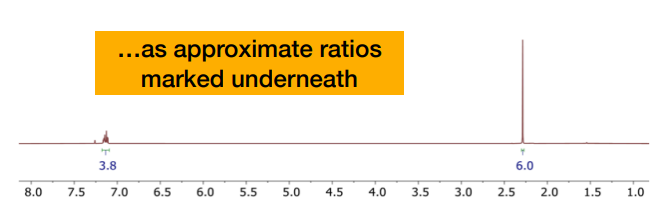
What is the 3.8 and the 6?
-the integrals presented as ratios
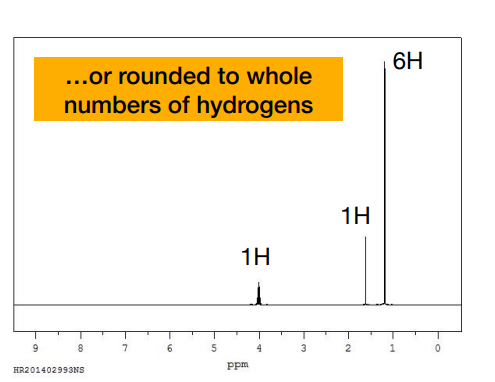
What is this?
-the integrals rounded to the nearest whole number
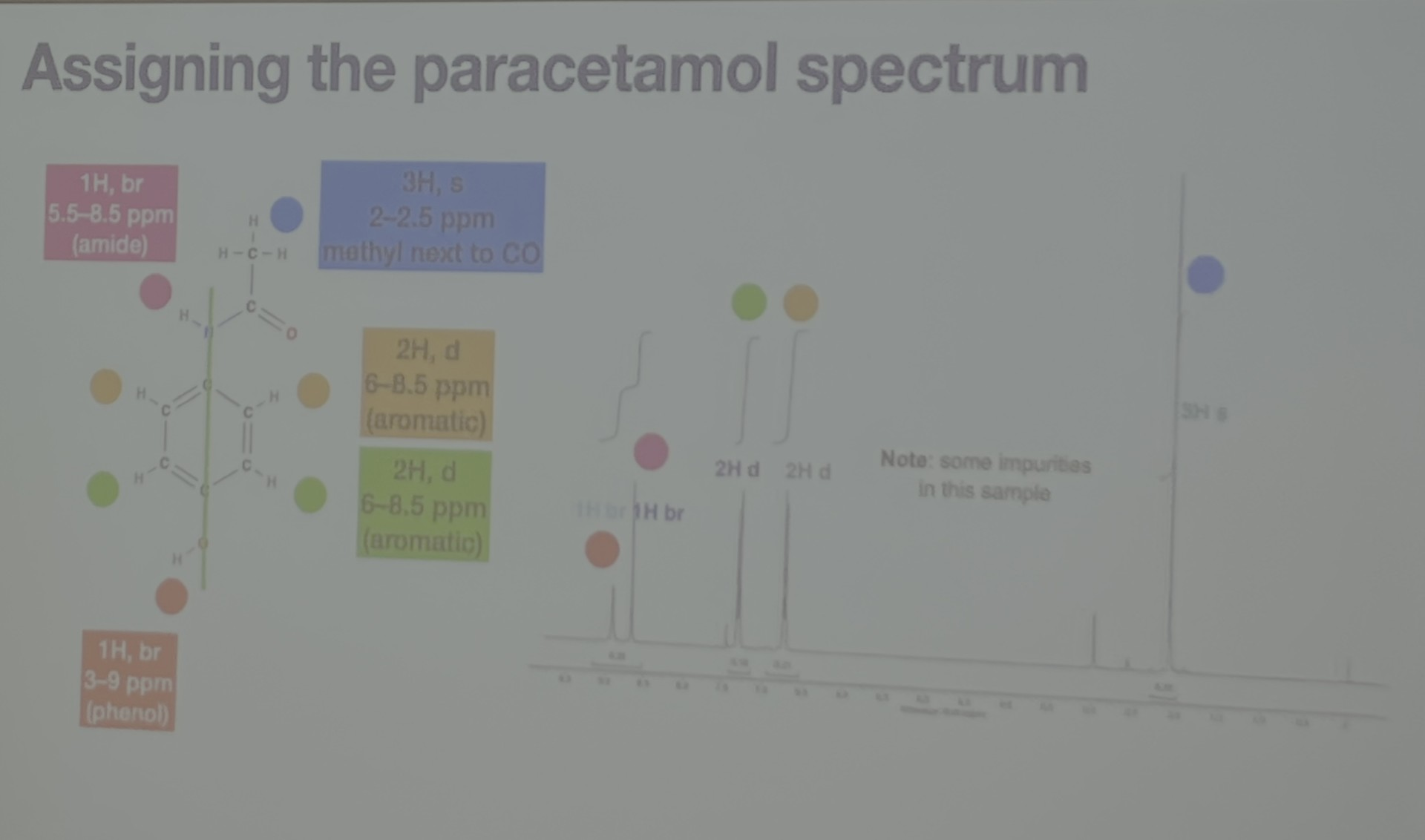
What is this image showing? How many different peaks would you expect?
the 3 proton environments of this molecule
-3 peaks because each chemical environment will always give a different 1H peak
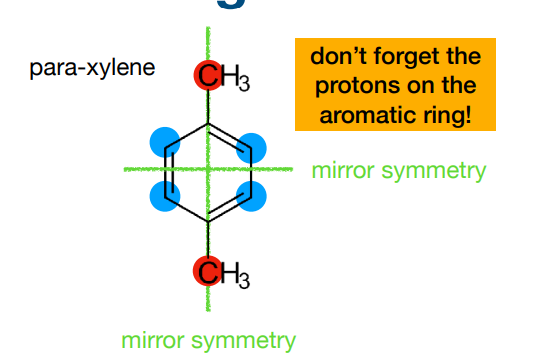
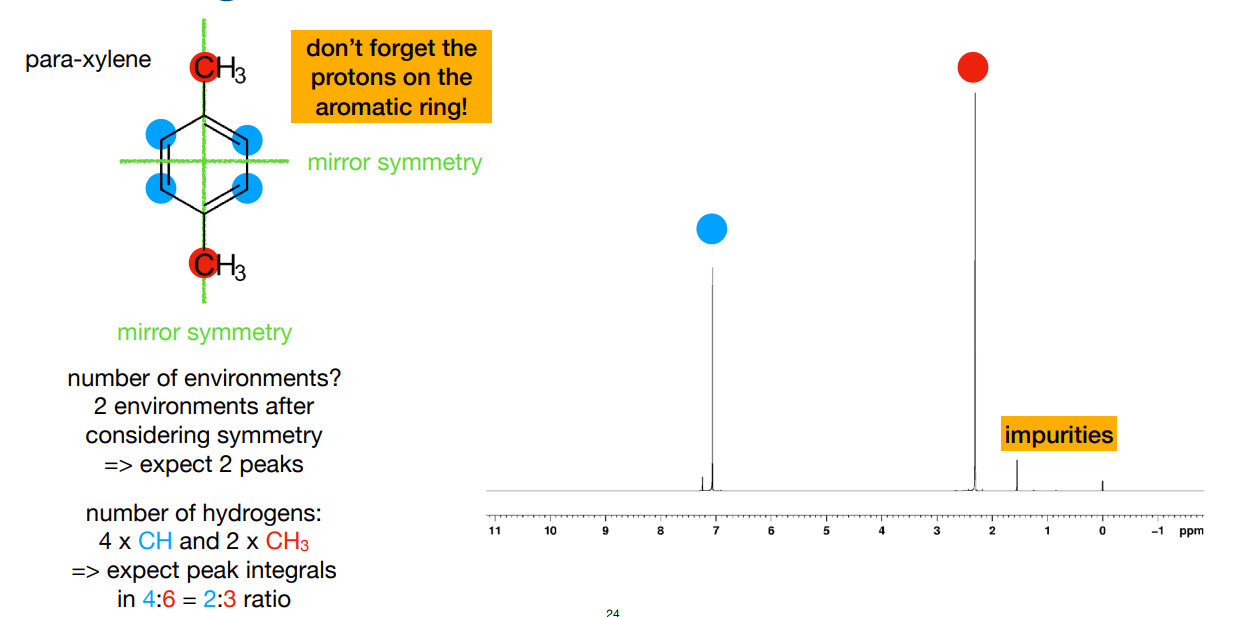
Why does this molecule only have 2 chemical environments? How many peaks would we expect? What peak integral would we expect?
-because symmetry cancels out
-2 peaks
-number of environments=number of peaks
-peak integral ratio shown in image
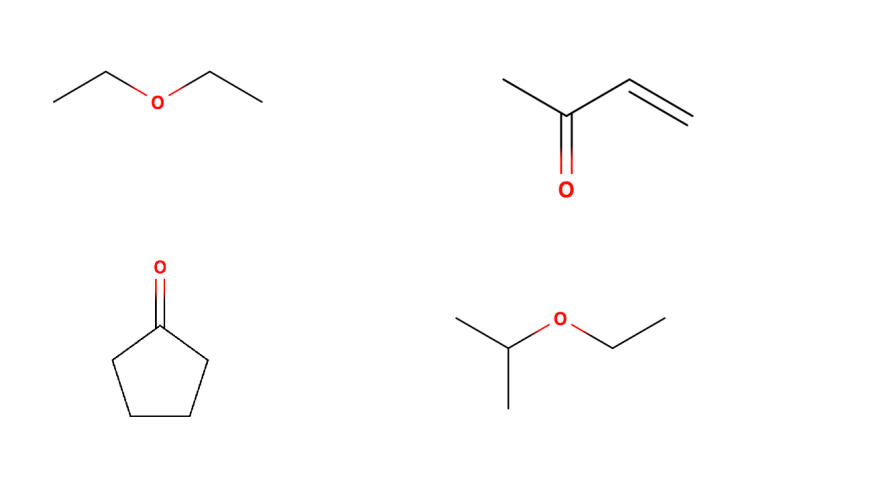
How many peaks do you expect, and what are their relative integrals? (number 1 is top, number 2 is bottom left)
2 environments, so 2 peaks
4 environments, so 4 peaks
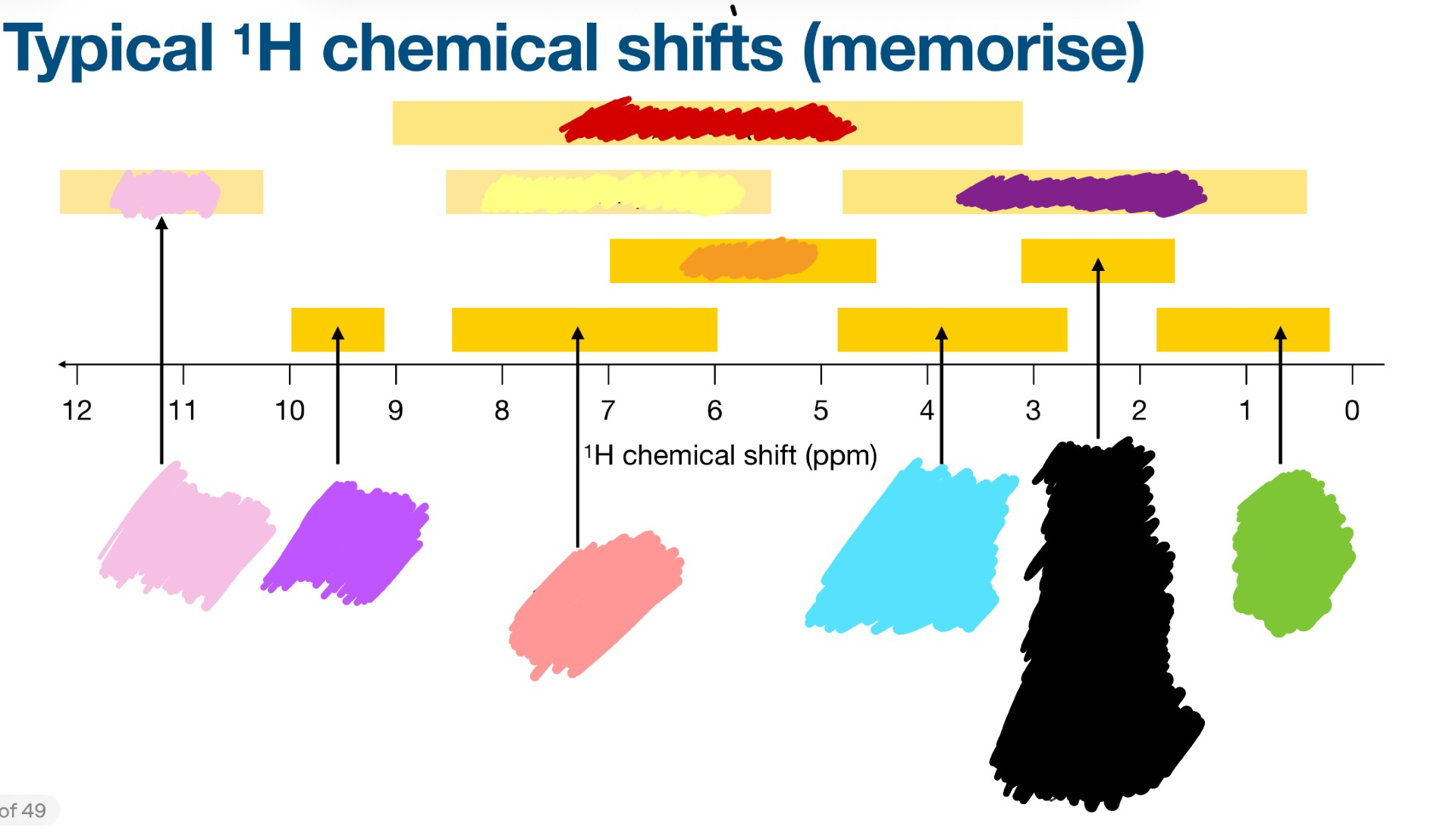
How are chemical shifts plotted? What greek letter represents chemical shifts?
δ
what do movement of electrons (charged particles) around nuclei create?
-opposing magnetic fields that shield nuclei from the external field
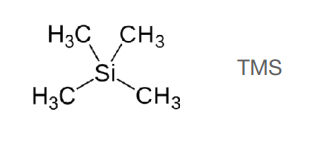
Why is TMS used as a reference for organic solvents? (2)
-because:
• symmetric molecule – it has only one NMR signal
the shielding effect of electropositive silicon creates a unique chemical environment that is unlikely to overlap with other peaks of interest
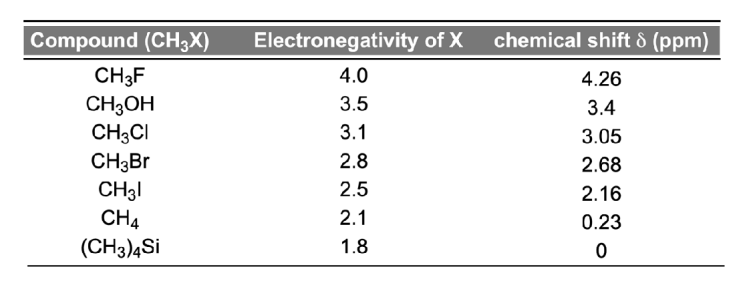
What effect do electron withdrawing groups have on shielding and chemical shifts?
-Shielding is caused by the electrons surrounding a nucleus.
-Electron withdrawing groups can decrease the electron density surrounding the nucleus. This deshields the nucleus, resulting in a larger chemical shift.
-1H chemical shifts correlate with the electronegativity of nearby bonded atoms groups:
How does shielding relate to chemical shifts?
-shielding refers to how much the local electrons around a nucleus protect it from the external magnetic field B0B_0B0.
Shielded proton → feels a smaller effective magnetic field → resonates upfield (lower δ value).
Deshielded proton → feels a larger effective magnetic field → resonates downfield (higher δ value).
-So the chemical shift (δ) tells you how shielded or deshielded a proton is.
How does ring current effect nuclear shielding and therefore chemical shifts?
-Aromatic ring currents cause magnetic shielding effects that depend on position relative to the ring.
protons outside the aromatic ring= deshielded, so downfield, so high δ so peak further on the left)
protons inside the ring= shielded, so upfield, so low δ or even negative)
What are “exchangeable” or “labile” protons? Why do they show variable chemical shifts?
These are hydrogens attached to electronegative atoms, such as:
–OH (the H in OH) (in alcohols, carboxylic acids)
–NH (the H in NH) (in amines, amides)
–SH (the H in SH) (in thiols)
-Because they can form hydrogen bonds that vary in strength depending on temperature, solvent, concentration, and acidity. These differences in hydrogen bon strength cause varying degrees of deshielding, so their chemical shifts cover a wide range.
What happens to a proton when it forms a hydrogen bond?
-It becomes deshielded because electron density is pulled away toward the electronegative atom, leading to a higher chemical shift (further left on the spectrum).
Why are –OH and –NH peaks broad in NMR spectra?
-Because hydrogen bonds form and break rapidly, and proton exchange between molecules occurs on the NMR timescale, which averages the signals and causes broadening.
What conclusion can be drawn if a peak disappears after adding D₂O?
-The missing signal corresponds to an exchangeable proton such as –OH or –NH.
Why is integration (i.e. area) of –OH and –NH peaks often inaccurate?
-The rapid exchange and broad nature of the signal distort its area, so the integral does not reliably correspond to the number of hydrogens. (so marked as ‘br’ for broad)
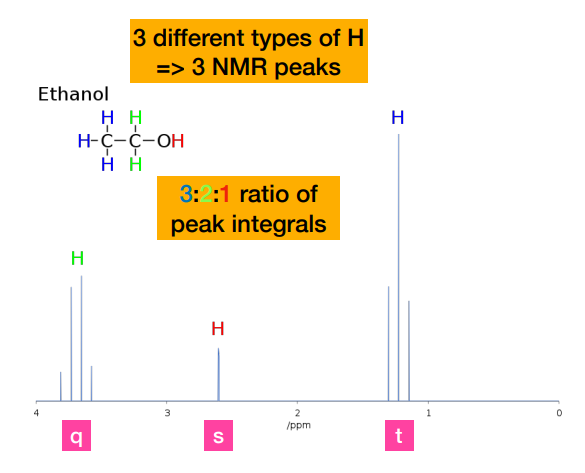
Why are some peaks in the NMR spectrum of ethanol split into multiple lines?
-These are called ‘multiplets’ and depend on the presence of other protons nearby. This can tell us a lot about the structure of a molecule!
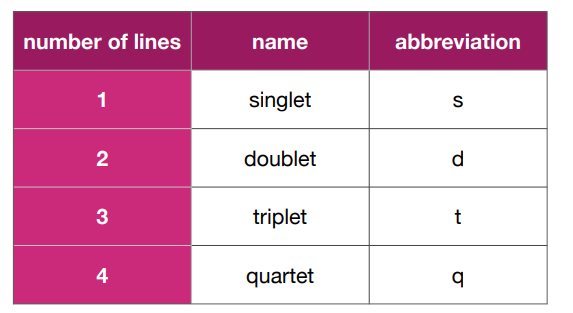
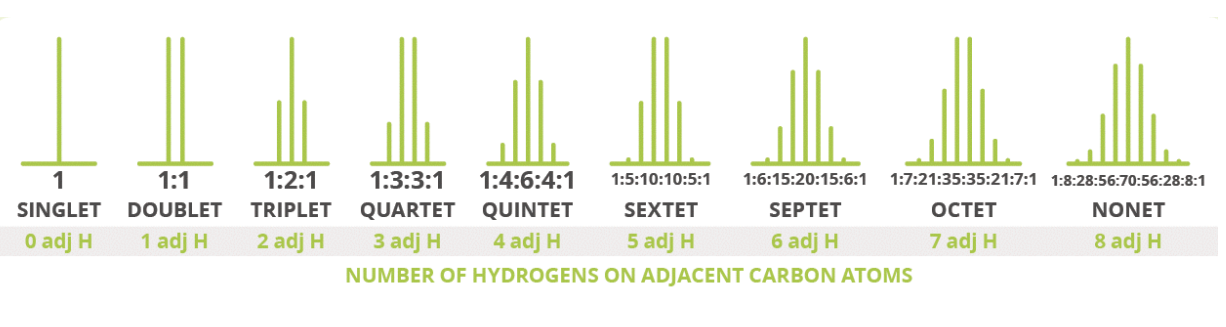
What are the different lines of a peak called and what is their abbreviation?
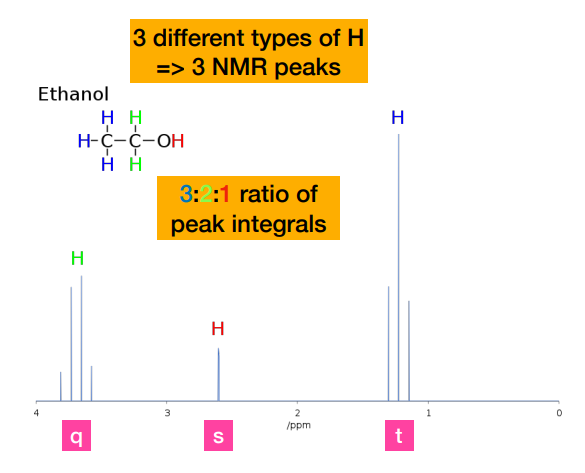
What do integrals (i.e. area under the peak tell you?)
-integrals tell you how many hydrogens contribute to each signal/chemical environment.
-CH₃ → 3 hydrogens → largest area
-CH₂ → 2 hydrogens → medium area
-OH → 1 hydrogen → smallest area
-NOT related to position on chemical shift. Position depends on functional group
What is scalar coupling aka J coupling?
-arises when protons in one environment are ‘near’ protons in another environment and can sense the state of these other spins. The effect is mediated by electrons, and so is transferred through chemical bonds.
-It causes one NMR signal to split into multiple peaks e.g. doublets, triplets and quartets
What are the rules for scalar coupling?
-Coupling between equivalent atoms is not observed e.g. a proton in a methyl group will not show couplings to the two other protons
-Coupling across four bonds or more is unlikely to be observed
-Coupling is symmetric – if A is coupled to B, B will also be coupled to A (with the same coupling constant) but not necessarily the same multiplicity – this depends on the number of protons
-Coupling is usually not observed to hydroxy or amine protons (because they exchange rapidly with solvent)
What is the rule for number of peaks i.e. scalar coupling?
-The OH proton usually doesn’t couple (it exchanges too fast) → singlet.
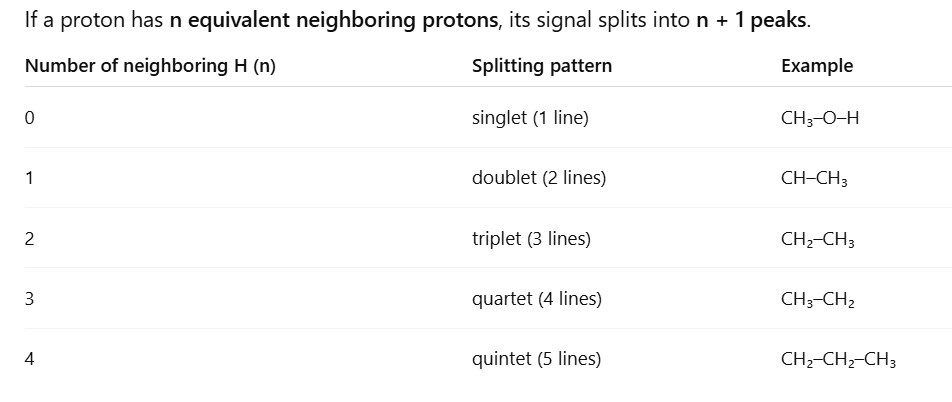

State their peak name, abbreviation and number of coupled spins
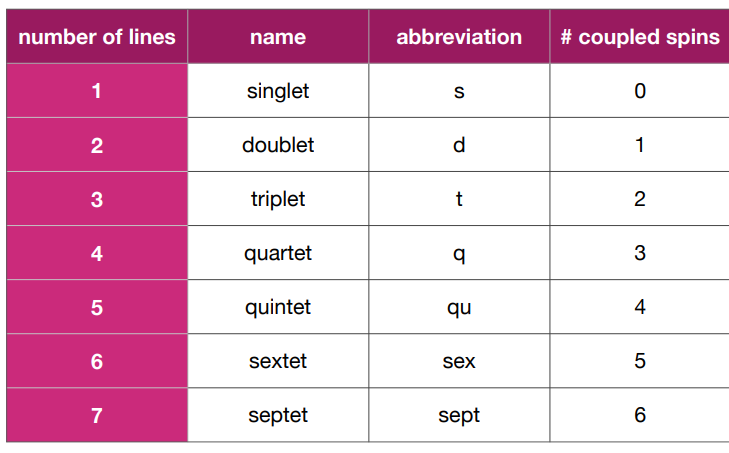
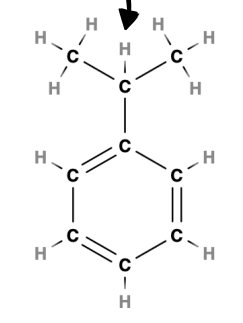
What is the coupling in this molecule?
simplified rule for coupling and coupling ratio i.e. number of peaks
-only refers to the protons attached to the directly adjacent carbon atom(s)
Describe the coupling in the methyl protons of ethanol
-Methyl protons are coupled to the two equivalent -CH2- protons, resulting in a 1:2:1 triplet. (referring to the one on the very right)
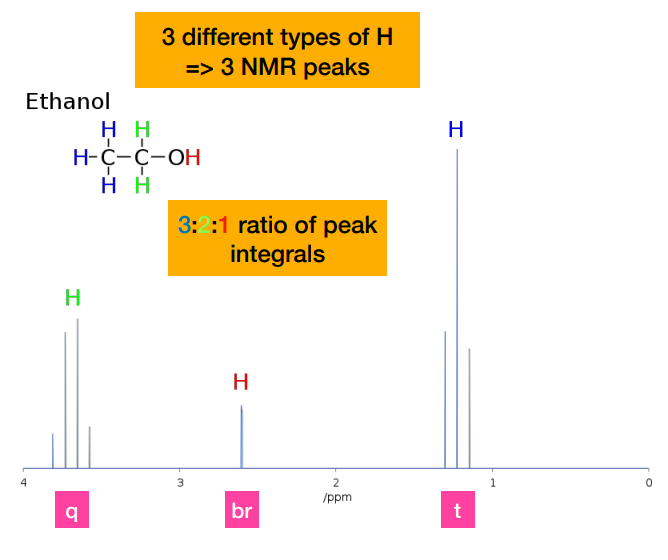
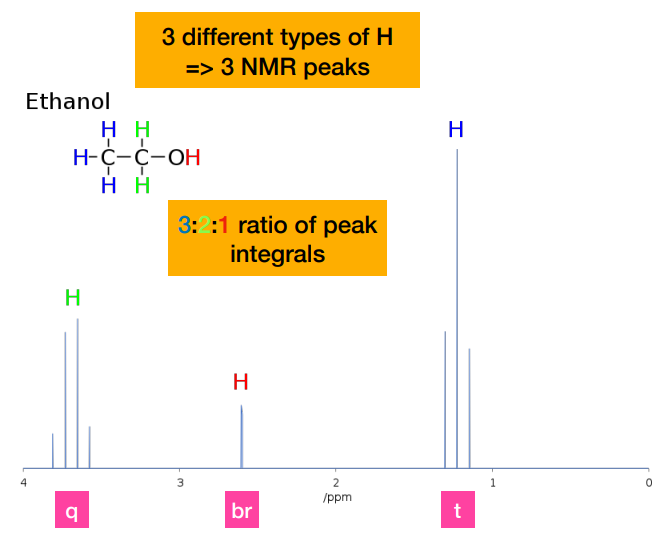
Why is there no coupling (i.e. peaks) of the hydroxyl proton?
-Coupling is not observed to a hydroxyl proton. The hydroxyl proton does not couple to the nearby -CH2- protons due to its rapid exchange with the solvent, so appears as a broad singlet (br)
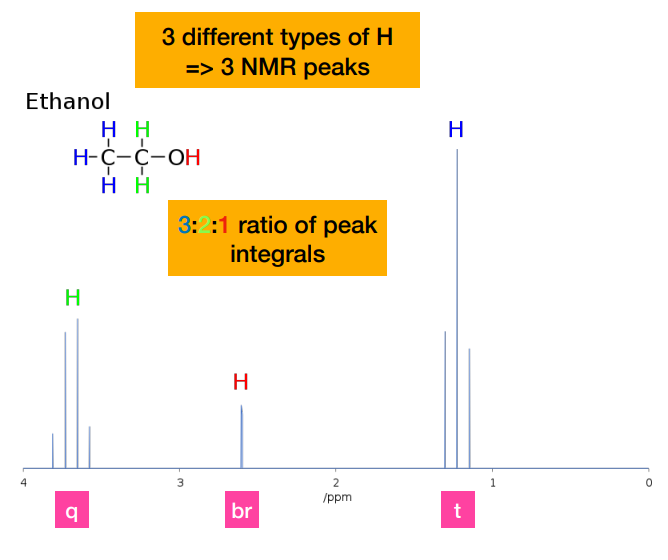
How do we know that he peak on the very left is the CH2 protons?
-Methyl protons are coupled to the two equivalent -CH2- protons, resulting in a 1:2:1 triplet. This ratio is NOT the integral peak ratio. Integral peak ratio relates to number of protons
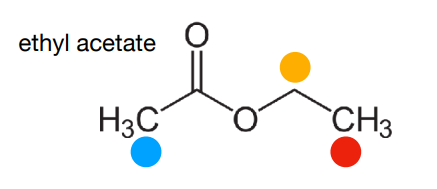
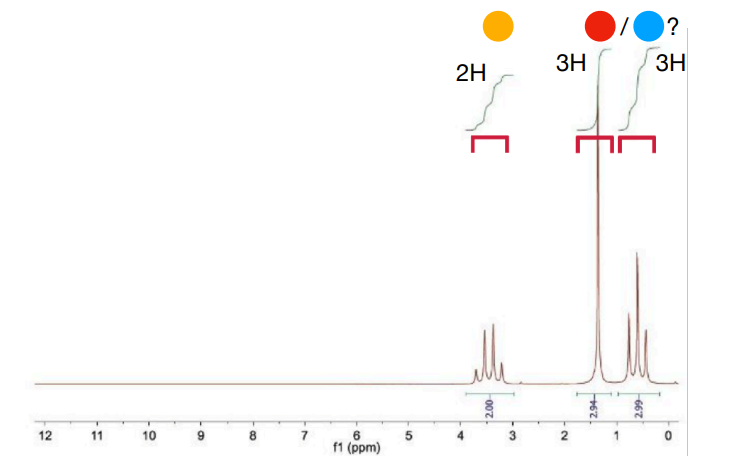
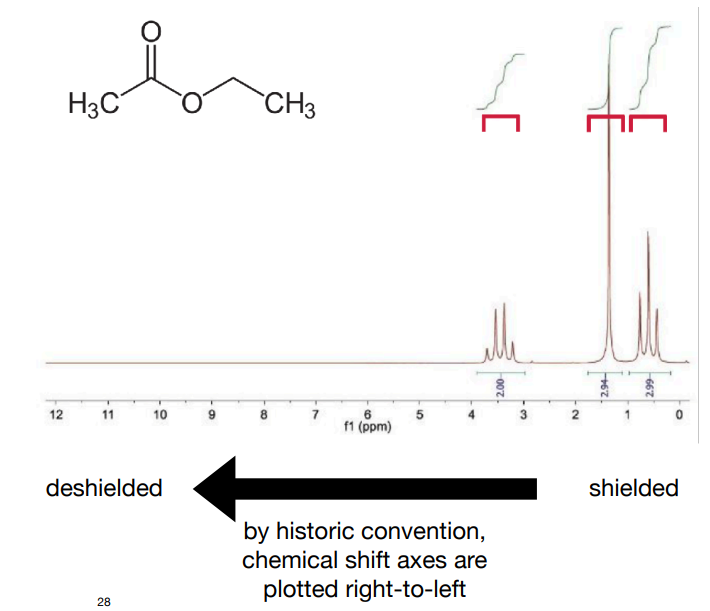
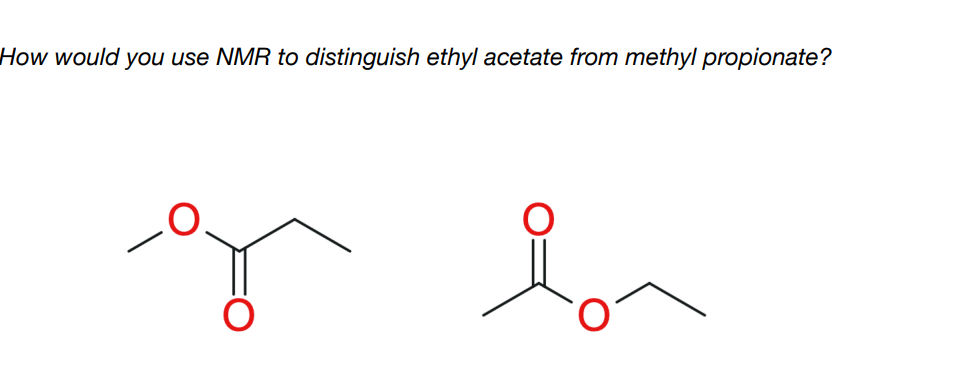
How would ethyl acetate look like on NMR? Mention the coupling peaks it would produce, why it would produce these peaks, and peak integral ratio
-integral ratio is 3:2:3
-Methyl protons within the ethyl group are coupled to the two nearby equivalent -CH2- protons, so appear as a 1:2:1 triplet.
-CH2 protons are coupled to the three nearby equivalent methyl protons. Coupling is not observed to the more distant methyl group. This results in a 1:3:3:1 quartet.
-The protons in the other methyl group are not near any other protons, so appear as a singlet.
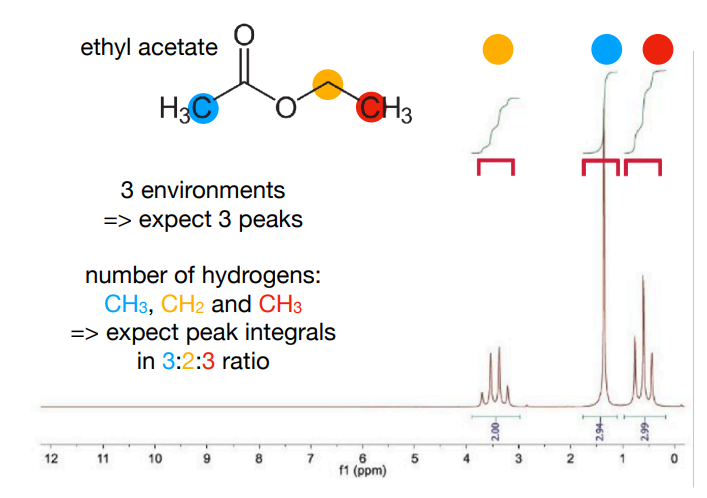
for 1. 3 peaks. 1 singlet, 1 quartet and 1 triplet (preferably say in order in structure or in order it would be in chemical shift)
-remember, symmetry counts as 1 not 2
If NMR has extra peak(s) then there should be, what does that mean?
-impurities in the sample
If you are given the molecular structure of a molecule, how do you know where it goes on the graph?
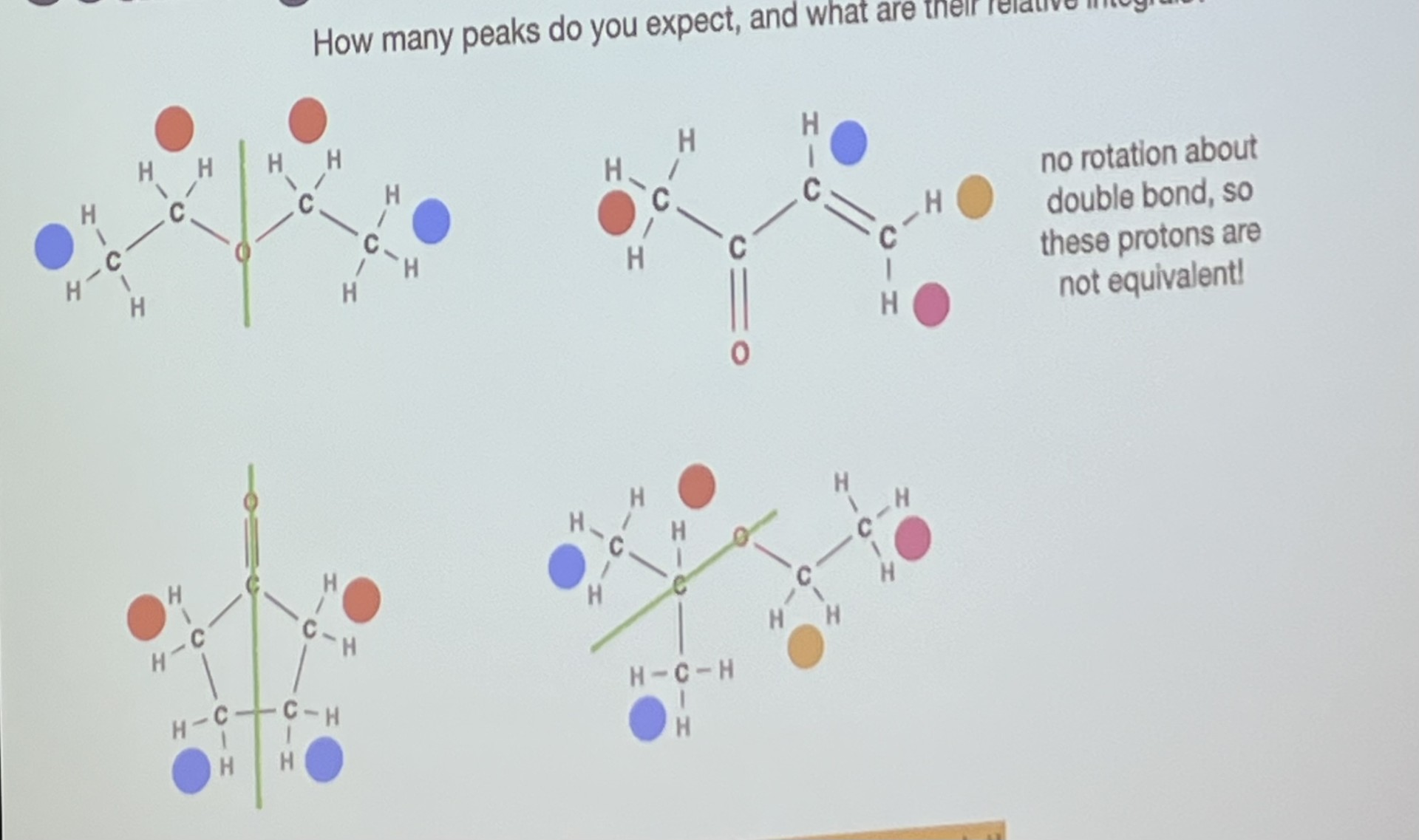
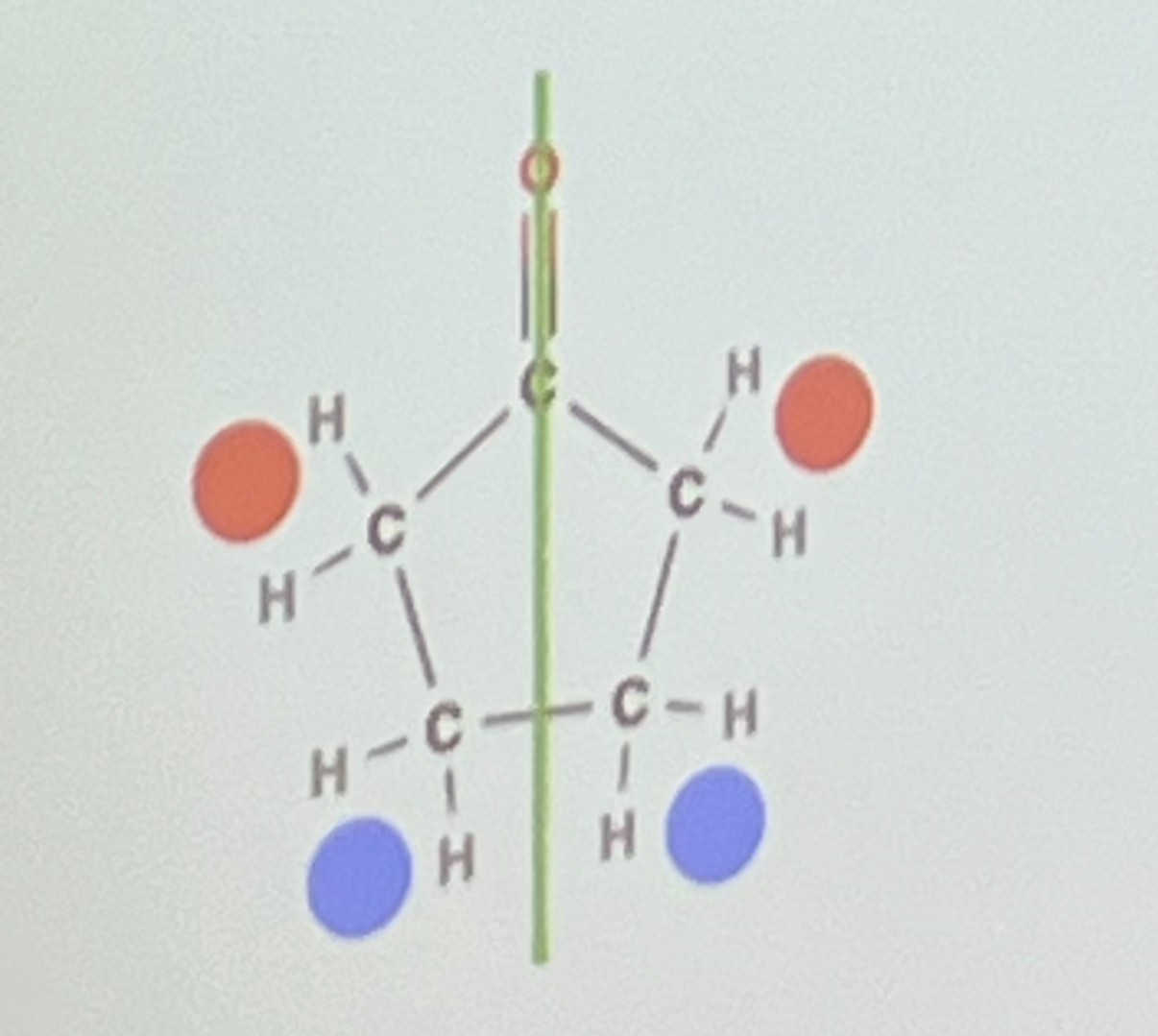
Why does this molecule only have 4 proton environments?
-because there is no rotation about the double bond, so these protons are not equivalent
Why does this molecule only have 2 proton environments?
-because mirror symmetry cancels out
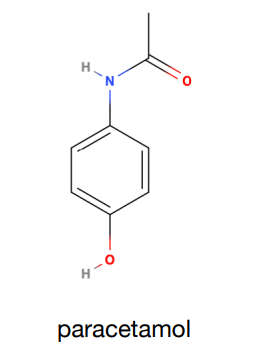
How many peaks would paracetamol produce in a proton NMR?
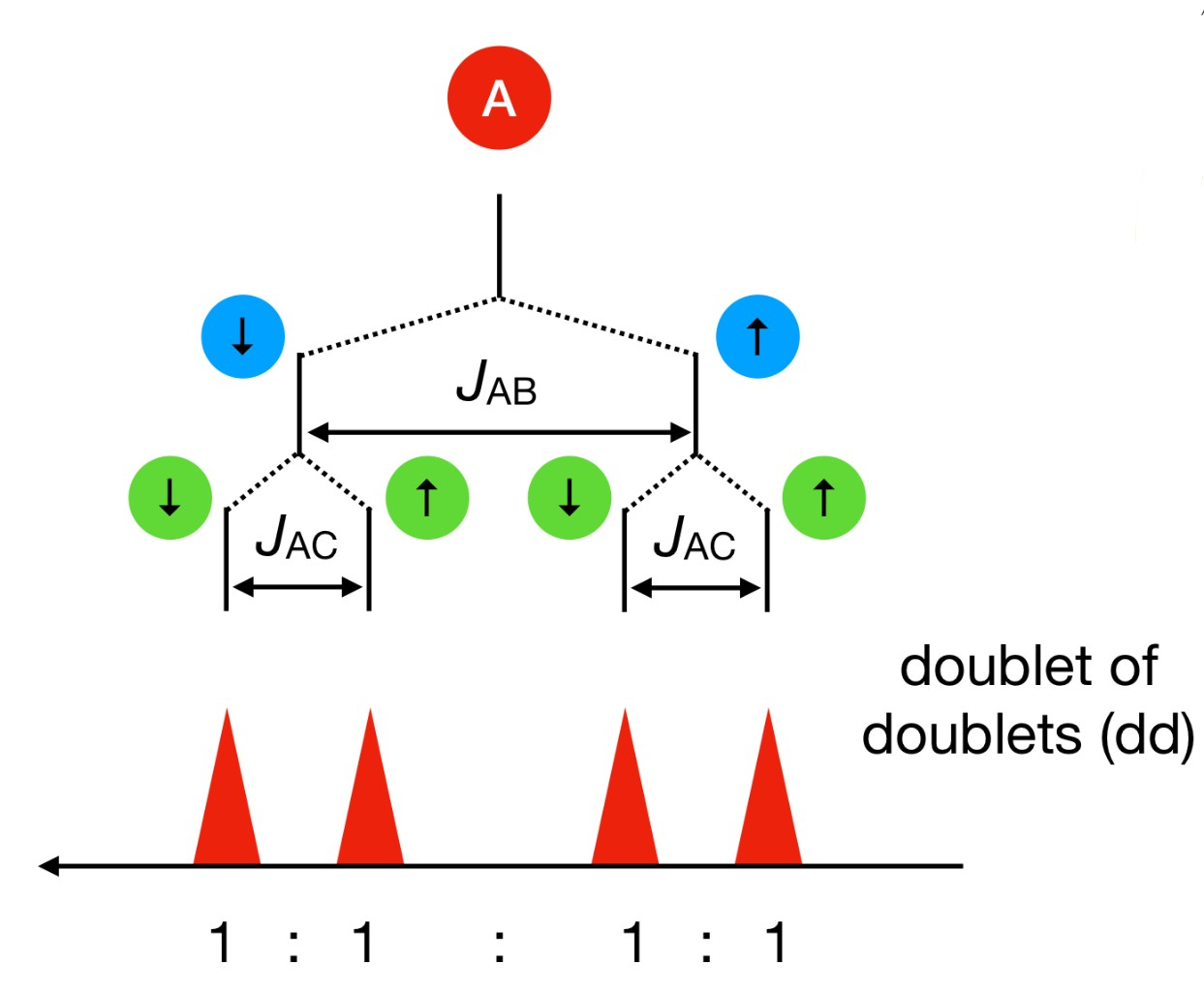
What happens when non-equivalent spins couple?
-non-equivalent spins will have different coupling constants, so coupling results in patterns like:
doublet of doublets (dd)
doublet of triplets (dt)
doublet of doublet of doublets
-the order you work out the couple doesn’t matter
When assigning peaks to a spectrum, how do you do it?
-Answers must account for:
chemical shift
multiplicity (coupling)
integration!
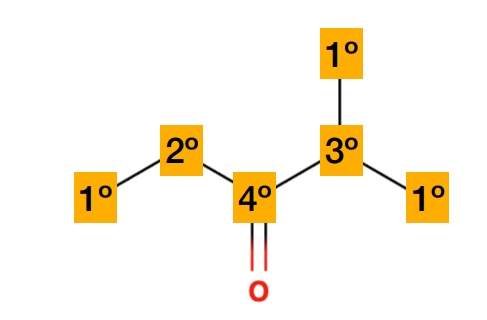
Carbon environments are commonly referred to as what?
-1º = primary = 1 bond to heavy atoms (CH3)
-2º = secondary = 2 bonds to heavy atoms (CH2)
-3º = tertiary = 3 bonds to heavy atoms (CH)
-4º = quaternary= 4 bonds to heavy atoms (C, no bonded H)
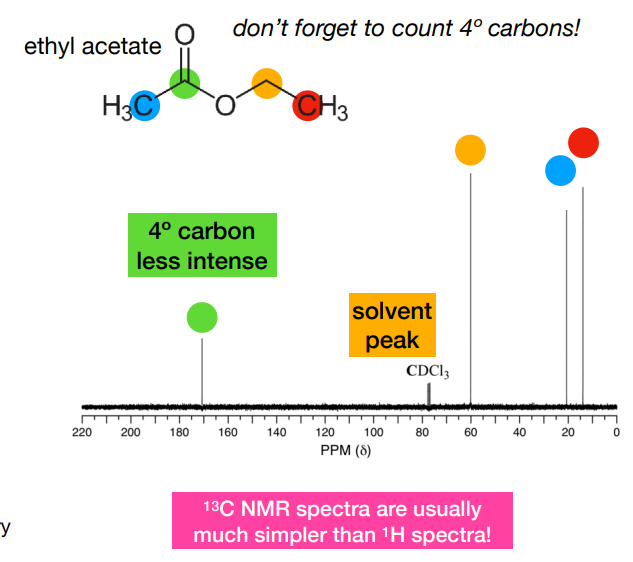
What information is in a proton (13C) NMR spectrum ?
-Number of peaks:
• the number of distinct carbon chemical environments
• related to molecular symmetry
-Area of peaks:
• 13C spectra are usually not quantitative
• integrals are not proportional to the number of atoms
• quaternary carbons (without attached 1H) are less intense
-Position of peaks:
• 13C has a wide range of chemical shifts (over 200 ppm) • depends on the functional group
-Shape of peaks:
• 13C spectra do not show coupling to other carbons
• Usually 1H couplings are also removed (‘decoupled experiments’)
• couplings to other nuclei like 19F can appear, and may be very large
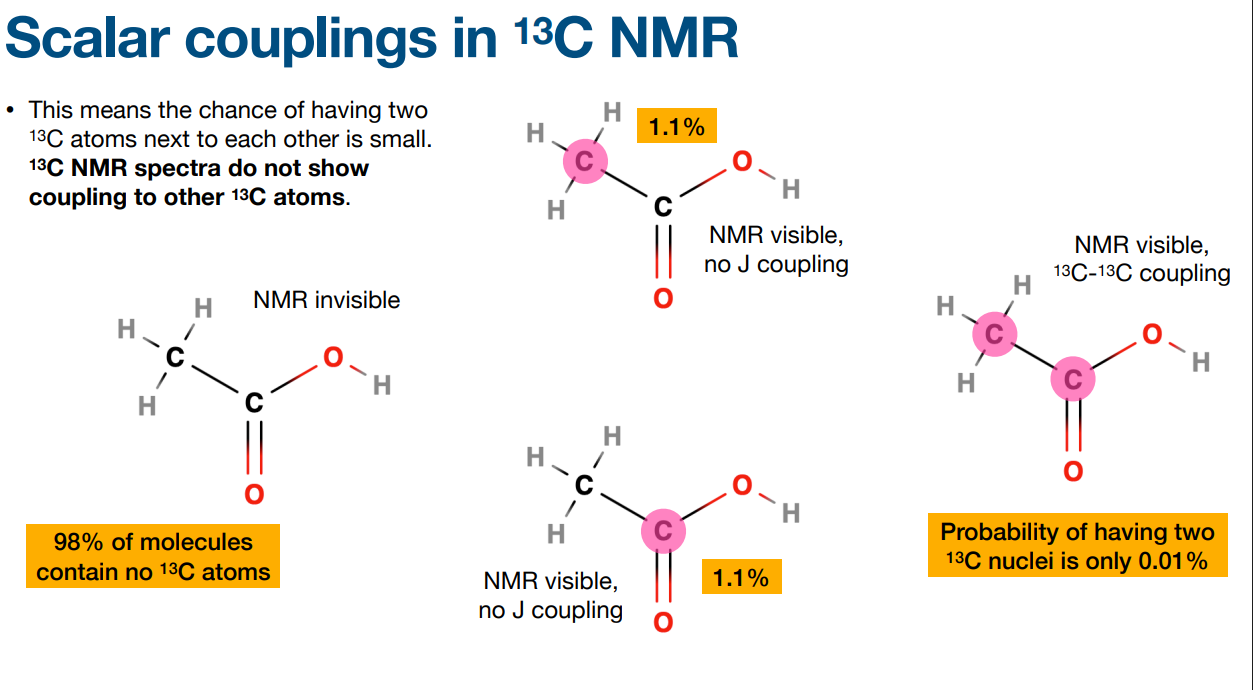
Why is some NMR visible and some not?
-due to the presence or absence of the 13C isotope in the molecule.
-Carbon atoms normally exist as 12C, which has no nuclear spin and is therefore NMR inactive. Only the 13C isotope has a nuclear spin (and is NMR active.
-You figure out if a molecule is "NMR visible" or "NMR invisible" by determining which carbon isotopes are present.
The chance of having two 13C atoms next to each other is…
-small
-13C NMR spectra do not show coupling to other 13C atoms.
-13C NMR spectra are also usually acquired in a way that removes couplings to 1H atoms – this is called decoupling. 13C multiplet is decoupled to 1H
State the regions of the 13C chemical shifts
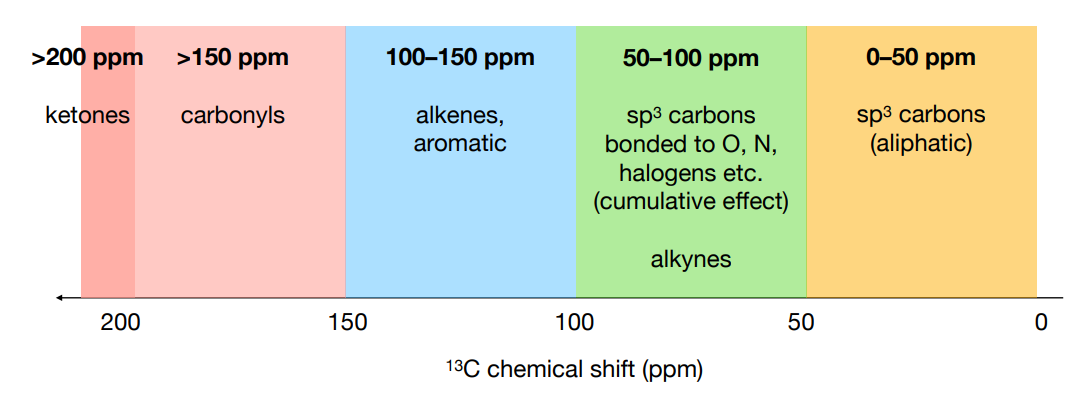
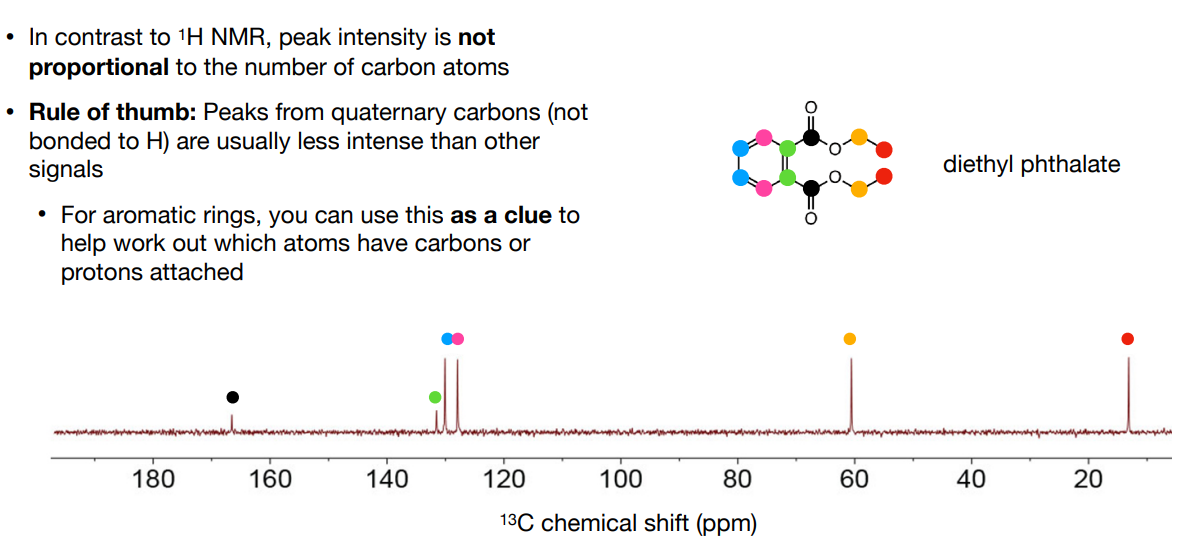
In 13C NMR chemical shifts, is the peak intensity/integral proportional to the number of carbon atoms like it is for 1H NMR?
-no
-Rule of thumb:
Peaks from quaternary carbons (not bonded to H) are usually less intense than other signals
For aromatic rings, you can use this as a clue to help work out which atoms have carbons or protons attached