Lecture 21: oxidation of fatty acids and ketone bodies
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
product of Beta oxidation
generates acetyl CoA during the pathway
-acetyl CoA enters TCA and produces more ATP
what coenzymes are produced in fatty acid oxidation?
FADH2 and NADH
How are ketone bodies formed?
in liver, acetyl-CoA produced by Beta oxidation is converted to ketone bodies, which are released into blood as a fuel source for other tissues
what organ CANNOT use ketone bodies?
the liver, bc it lacks enzymes
what are the fates of fatty acyl CoA and what are their purposes
-Beta oxidation ketogenesis for energy
-triacylglycerols for storage
-phospho/sphinigolipids for membrane lipids
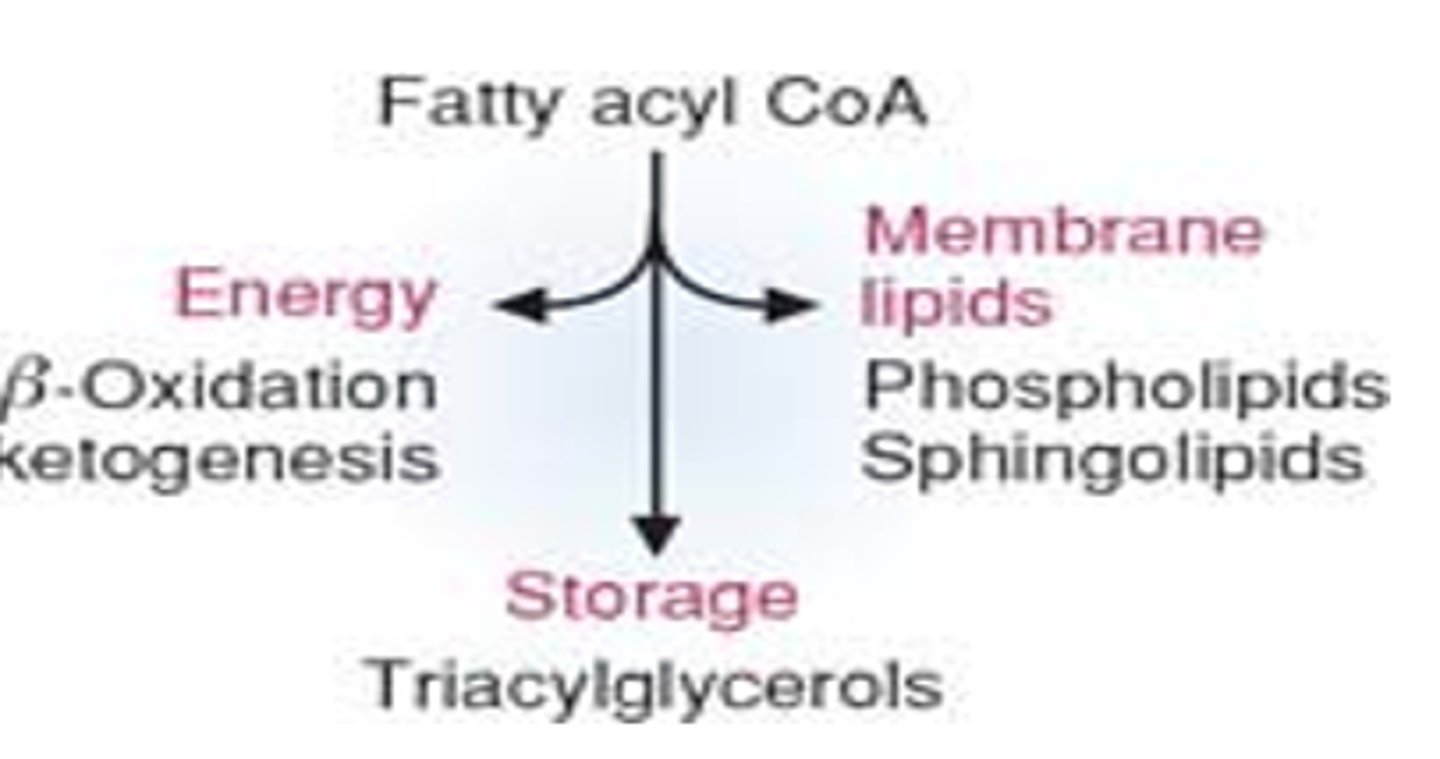
beta oxidation of fatty acids
-major pathway in the mitochondria
-generates an acetyl CoA which then enters TCA to produce more energy, or it goes into ketone body production
which tissues oxidize FA?
-many tissues, including muscle, oxidize FA to CO2 and H2O
-brain and nervous tissue might as a last resort (blood brain barrier)
-RBCs cannot bc they lack mitochondria
where are ketone bodies used as energy? where can ketone bodies not be used for energy?
muscle and kidney
-potentially brain if concenntration is high
-liver CANNOT use ketone bodies since it lacks enzyme for their activation (this conserves energy)
short chain fatty acids
2-3 carbons
-used in gut and can cross blood brain barrier
-fairly soluble
medium chain fatty acids
4-12 carbons
long chain fatty acids
12-20 carbons
-predominant form in the body
Very long chain fatty acids
more than 20 carbons
FA longer than 4 Carabons require
being carried in blood by albumin
-this is because they are hydrophobic
How are fatty acids activated?
-via fatty acyl CoA synthetase, fatty becomes a fatty acyl AMP
-hydrolysis of pyrophosphate provides energy to make fatty acyl CoA and generates 2Pi, making the activation irreversible
what is the activate form of fatty acids?
fatty acyl CoA
-FA bound to CoA
where are short chain FAs activated?
cytosol or mitochondria
where are medium chain FAs activated?
they cross the inner mitochondrial membrane and are activated in the mitochondrial matrix
where are long chain FAs activated?
-activated by enzymes in the ER membrane, inner mitochondrial membrane, and peroxismal membrane
How is a long chain fatty acid transported to the mitochondrial matrix?
-an enzyme on the outer mitochondrial membrane catalyzes the transfer of FA from CoA to carnitine
-carnitine is the carrier for long chain FAs into mito matrix
-translocase on inner mitomembrane transports fatty acyl carnitine

what happens once the long chain fatty acid is inside the mito matrix?
-a second enzyme transfers FA from fatty acylcarnitine back onto CoA
-this supplies a substrate for beta oxidation!
Steps of fatty acid oxidation
-first three steps are similar to TCA cycle between succinate and oxaloacetate
-4th step produce FADH2, NADH, and acetyl CoA
-process repeats until all the carbons of a FA are converted to acetyl CoA, two carbons at a time
enzymes in beta oxidation
variable for tissue type and fatty acid chain length
-enzymes needed for oxidation will change as the length of the FA chain gets shorter
how much ATP is made per 2C units oxidized?
12 mols ATP
-terminal two carbon is produced as acetyl CoA
why do unsaturated fatty acids produce less ATP?
-presence of double bonds reduces amount of ATP produced because of different requirements for NADH and FADH2 production
-no longer a need for FAD, so you lose those electrons that would have gone to ETC
how much ATP can be produced per mole of palmitate oxidized? (16C)
about 100 ATP
why are newly synthesized fatty acids not immediately transported to the mitochondrial matrix?
-NADH and FADH2 levels are used to regulate FA oxiidation
-if ATP levels are high, ETC/OxPhos will slow down and NADH levels will rise, inhibiting FA oxidation
beta oxidation of odd numbered fatty acids
-same processas even chains until the final 5 carbons remain
-the final reaction generates 1 acetyl CoA and one propionyl CoA
what is propionyl CoA?
3 carbon fatty acyl CoA produced in final step of beta oxidation of odd numbered fatty acids
how come propionyl CoA can produce glucose?
propionyl CoA is carboxylated to methylmalonyl CoA, which is converted to succinyl CoA, that goes into the TCA
what is the correct form of unsaturated fatty acids to be oxidized?
trans double bond between alpha and beta carbons
-often rearranged to accomodate this by isomerases
Oxidation of very long chain fatty acids in peroxisomes
-donate electrons to O2 via FADH2 (no ATP made)
-H2O2 is made and degraded by catalase
-make octanoyl CoA to move into the mitochondrion for oxidation via carnitine shuttle
unlike fatty acid oxidation in the mitochondria, VLCFA do not use
CPT1/CPT2
what metabolic conditions favor ketone body formation?
-high levels of fatty acids during fasting, low carbs; high acetyl CoA
-high FA leads to accumulation of acetyl CoA, high NADH
-glucagno
-liver is synthesizing glucose by gluconeogenesis
-high NADH drives OAA to malate, making less OAA available for conversion to citrate, so acetyl CoA accumulates
after how many days of fasting are KB used by the brain?
5 days, give or take
-KBs first used in muscles over short fasts
what are the three ketone bodies produced in the liver?
-B-hydroxybutyrate (most prevalent in the blood)
-free fatty acids
-acetoacetate
formation of acetoacetate
formed by combination of two acetylCoAs and D-B hydroxybutyrate
-leads to formation of ketone bodies
advantages of beta-hydroxybutarate to acetoacetate
-being reduced, it can regenerate and NADH in target tissue
-prevents acetone formation
-produces more energy
what are the advantages of using the energy from ketone body formation instead of simply oxidizing FAs all the way to acetyl CoA?
Energy produce is nearly the same, but oxidation of ketone bodies is advantageous bc
-it can be used by many tissues as fuel
-decreases use of muscle protein amino acids for source of glucose production
relationship of FA and pyruvate dehydrogenase
-high FA shuts down PDH
-pyruvate can't enter TCA cycle, glycolysis intermediates build up
-therefore, FA oxidatino becomes the key energy producer
following a high carb meal, FA synthesis
-is turned ON
-malonyl CoA shuts down CPT1
-no FA oxidation, no production oof ketone bodies
during fasting, FA synthesis is
-turned OFF
-low malonyl CoA, carnitine shuttle operative
-OAA and malate aare directed towards glucose production
-ketone bodies produced