CC5C: Separations and Purifications
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
What goes in the distilling flask?
The mixture of compounds you want to separate out
Why do you need to put the distilling flask in an oil bath?
Because oil won’t evaporate when you heat it up and it’s good for maintaining a constant temperature throughout the distillation process
Why and how does the condenser stay cool?
How: water cycles in and out
Why: to condense the gas that has evaporated out of the distilling flask so that it ends up as a liquid in the receiving flask
Why do you need a vacuum in the distillation process?
Because this will lower the pressure of the entire system. Having a lower pressure makes it easier to vaporize substances (that might otherwise have a really high boiling point) because, at lower pressure, there isn’t much of a force pushing back on the liquid
Simple vs. fractional distillation
Simple distillation is better for separating compounds with very different boiling points
Fractional distillation is better for separating compounds with boiling points that are close together (<30°C difference)
Fractioning column
A column packed with beads to increase the surface area for vapor-liquid contact, which enhances the separation process by allowing for multiple vaporization-condensation cycles as the vapor rises through the column
Aqueous vs organic phase
Aqueous phase: contains water and ions
Organic phase: contains neutral compounds
How should you separate two compounds into an aqueous and an organic layer?
Add an acid or base to the mixture to make one of the compounds an ion, keeping the other neutral
How should you separate three compounds if two could be affected by an acid/base?
Add a weak acid/base so only the stronger acidic/basic compound will turn into an ion
What is chromatography?
An analytical technique commonly used for separating a mixture of chemical substances into its individual components, so that the individual components can be thoroughly analyzed
Mobile phase/carrier
Term used in column chromatography to describe the solvent moving through the column
Stationary phase/absorbent
Term used in column chromatography to describe the substance that stays fixed inside the column
Eluent
Term used in column chromatography to describe the fluid entering the column
Eluate
Term used in column chromatography to describe the fluid exiting the column (that is collected in flasks)
Elution
Term used in column chromatography to describe the process of washing out a compound through a column using a suitable solvent
Analyte
Term used in column chromatography to describe the mixture whose individual components have to be separated and analyzed
Why do we add a stationary phase and mobile phase to the column in column chromatography?
The different components of the analyte will exhibit varying degrees of affinities to the stationary phase and the mobile phase, so they’ll travel at different speeds through the stationary phase as the mobile phase flows through it. This allows the different components to separate out.
Normal-phase vs. reverse-phase chromatography
Normal phase: polar stationary phase and non-polar mobile phase
Reverse phase: non-polar stationary phase and polar mobile phase
Paper chromatography
Basis of separation: polarity of molecules
Process: compound spotted directly on a cellulose (stationary phase) paper. Mobile phase is liquid.
Thin layer chromatography (TLC)
Basis of separation: polarity of molecules
Process: glass is coated with thin layer of silica (solid stationary phase) that the compound is spotted onto. Mobile phase is liquid.
Liquid column chromatography
Basis of separation: polarity of molecules
Process: glass column is packed with slurry of silica (solid stationary phase). Mobile phase is liquid.
Size exclusion chromatography
Basis of separation: size of molecules
Process: small molecules get trapped in the pores of the stationary phase (silica beads), while large molecules flow through the gaps between the beads.
Ion-exchange chromatography
Basis of separation: ionic charge of the molecules
Process: molecules possessing the opposite charge as the resin (solid stationary phase) will bind tightly to the resin, and molecules having the same charge as the resin will flow through the column and elute out first.
Gas chromatography
Basis of separation: affinities for the stationary liquid phase and their volatilities (boiling points).
Process: compounds that have a greater affinity for the stationary phase will move more slowly through the column. Compounds with a lower boiling point will move faster through the column. Solid or liquid stationary phase. Inert carrier gas mobile phase.
Affinity chromatography
Basis of separation: specific interactions proteins have with a ligand
Process: The column is filled with a ligand that specifically binds to the target protein. Proteins that do not have an affinity for the ligand are eluted first. The target protein is then eluted by adding a solution that disrupts the protein-ligand interaction.
What is the retention factor (Rf) and how do you calculate it?
Rf is used in thin layer chromatography as a quantitative measure of the polarity of each component. Rf = distance travelled by individual component/total distance travelled by solvent (mobile phase)
Retention time
The time it takes for a particular component to pass through the column from the point of injection to detection
Gradient elution
A technique where the composition of the mobile phase is gradually changed during the separation process to improve the separation of components with similar properties
What is gel electrophoresis and how does it work?
It is a technique used to separate out DNA or proteins based on their size.
An electric field passes through the gel to get the bands to move. One size has the negative cathode (where reduction occurs) and the other side has the positive anode (where oxidation occurs). Bands start at cathode and go toward anode. Electrodes and gel is submerged in a buffer that conducts electricity.
Agarose vs. polyacrylamide (SDS-PAGE)
Agarose: used to separate big pieces of DNA (>50 bp)
Polyacrylamide: used to separate small DNA or protein
What does SDS do?
Denatures proteins, disrupting any non-covalent interactions they may have. this makes it so the charge of the proteins isn’t a factor when they’re separating out onto the gel
Enantiomers
Stereoisomers that are mirror images of each others but non-superimposable
Stereoisomers
Molecules that have the same molecule formula and the same connectivity of atoms, but they differ in the spatial arrangement of their atoms
Chiral column chromatography
Purpose: To separate enantiomers (resolution of enantiomers)
Process: Chiral stationary phase only binds to one enantiomer, so the other elutes faster
Gas chromatography can also be used
Racemic mixture
1:1 ratio of two enantiomers of a chiral molecule
500 mL of water (d = 1 g/mL) and 500 mL of dichloromethane (d = 1.3 g/mL) are added to a mixture containing benzoic acid, cresol, methoxyethane, and N-methylethanamine. Four solutions are available for extraction of the mixture: HCN, HCl, NaOH, and LiHCO3. After the initial wash, the top layer from each extraction was retained and one of the preceding solutions was added.
Which of the following statements most accurately describes this procedure?
A) Methoxyethane will only be extracted upon protonation into a carbocation.
B) Benzoic acid can be extracted and isolated with either LiHCO3 or NaOH.
C) After washing with LiHCO3 and then NaOH, only N-methylethanamine and methoxyethane remain in the separatory funnel.
D) Cresol must be extracted first with a strong mineral acid such as HCl.
B) Benzoic acid can be extracted and isolated with either LiHCO3 or NaOH.
An azeotrope is a mixture of liquids whose proportions cannot be changed by simple distillation because, when it is boiled, the vapor has the same proportions of constituents as the unboiled mixture.
From an azeotropic mixture of 95% ethanol and 5% water, which of the following methods would NOT allow for further purification of ethanol at the azeotropic point?
A) Addition of an agent like cyclohexane to the mixture to form a ternary azeotrope, followed by distillation
B) Addition of calcium oxide to the mixture, followed by filtration
C) Dissolution of a salt like potassium acetate into the mixture, followed by distillation
D) Distillation conducted in an all-nitrogen gas (N2) environment
D) Distillation conducted in an all-nitrogen gas (N2) environment
Nitrogen is an inert gas so it wouldn’t interact/change anything about the components of the mixture that would allow for further purification.
What solvent would be most effective at purifying through recrystallization a quantity of sugar contaminated with some table salt?
A) Petroleum ether
B) Ethanol
C) Water
D) Benzene
B) Ethanol
Ethanol dissolves sugar due to their similar polarities, allowing for hydrogen bonding between the two substances. Salt, on the other hand, is primarily ionic and has a strong ionic bond that is not easily disrupted by ethanol's polarity.
A) Petroleum ether wouldn’t dissolve either substance because it is nonpolar. Sugar is polar and salt is ionic
C) Water can dissolve both sugar through hydrogen bonding and sat through solvation so it wouldn’t be effective in recrystallization
D) Benzene is nonpolar
What is eluting power and what type of solvent has a higher eluting power?
Eluting power refers to a solvent's ability to carry compounds through the stationary phase, with stronger eluting power leading to faster movement
Polar solvents have a higher eluting power than non-polar solvents
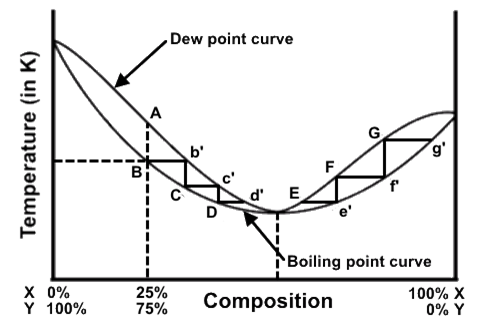
A distillation procedure is performed with a starting mixture of 25% X and 75% Y, and the distillation steps have been superimposed onto the liquid-vapor phase diagram.
A) Heating a 25% X : 75% Y mixture to temperature B will generate a vapor of a composition A over a liquid of a composition B.
B) At a composition D, the distillate will boil at a lower temperature because it has a lower percentage of X than the original mixture.
C) During a distillation of a 25% X : 75% Y mixture, the procedure will progress from point B to point G and beyond until the mixture is 100% X.
D) Point B is the boiling point of the original mixture at a composition B, and the vapor that is collected at that temperature has composition b’.
D) Point B is the boiling point of the original mixture at a composition B, and the vapor that is collected at that temperature has composition b’.
What combination of relative polarities of the absorbent and eluent causes the dots to barely move, move halfway up the plate, and move all the way up the plate?
Barely any movement: sticky absorbent, weak eluent
Halfway up: moderately sticky absorbent, moderately strong eluent
All the way up: non-sticky absorbent, strong eluent