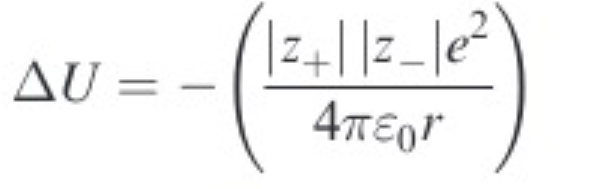Inorganic Chem test 2
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
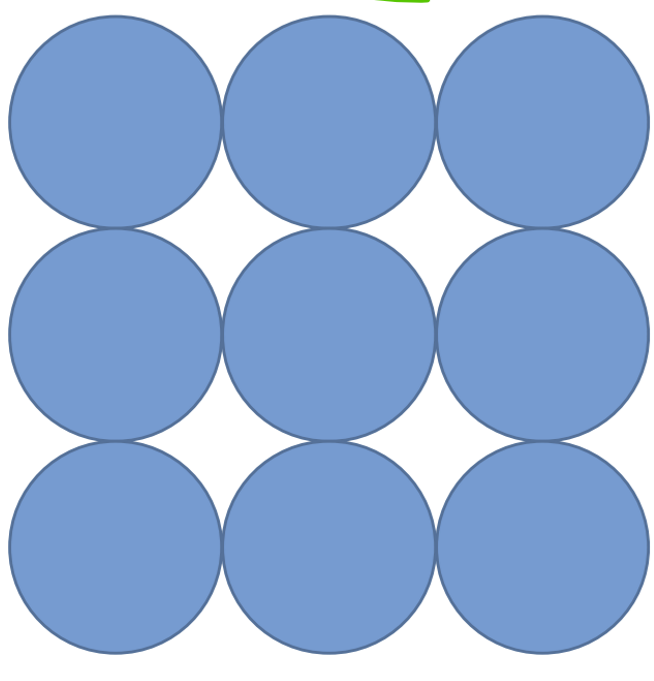
Close packing that maximizes interatomic contact
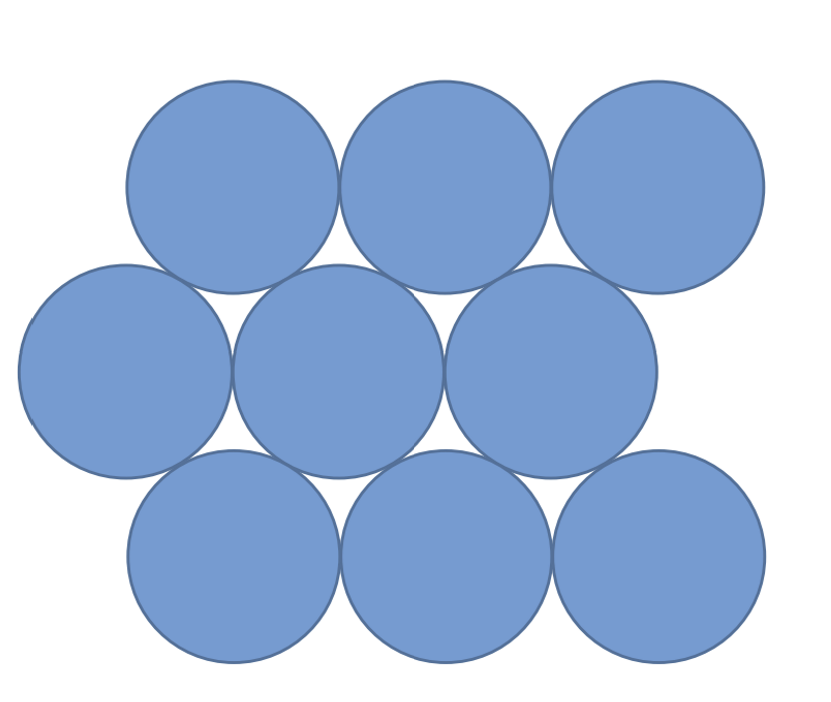
Close packing that minimizes interatomic contact
Hexagonal close packing
6 spheres surround each, so coordinate number is 6
Cubic Close packing
ABCABC
12 neighbors
Coordinate number = 12
Hexagonal close packing
ABAB
12 neighbors
Coordinate number = 12
Unit Cell
most basic and least volume consuming repeating structure of any solid
repeats itself = lattice
Can be cubic close packed (face centered packed)
hexagonal close packed
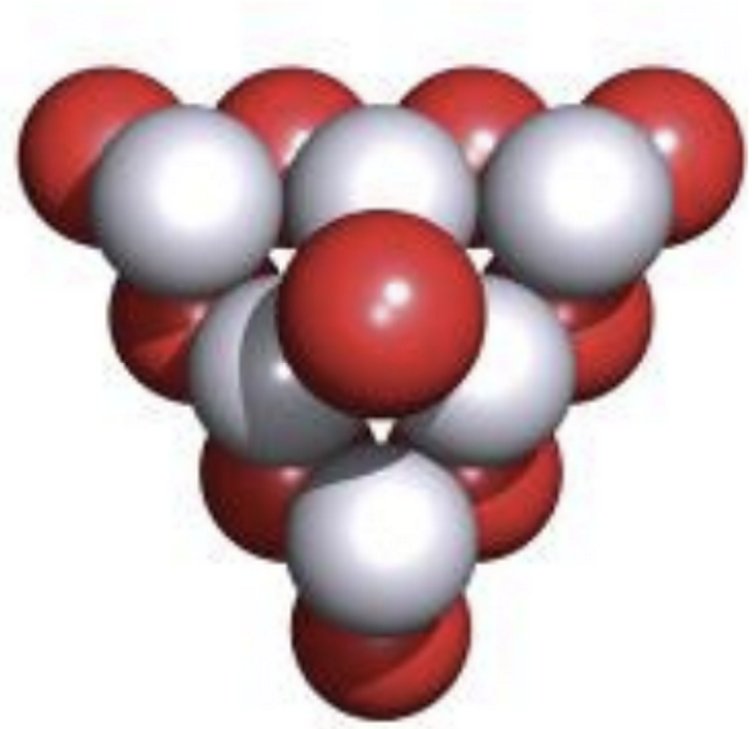
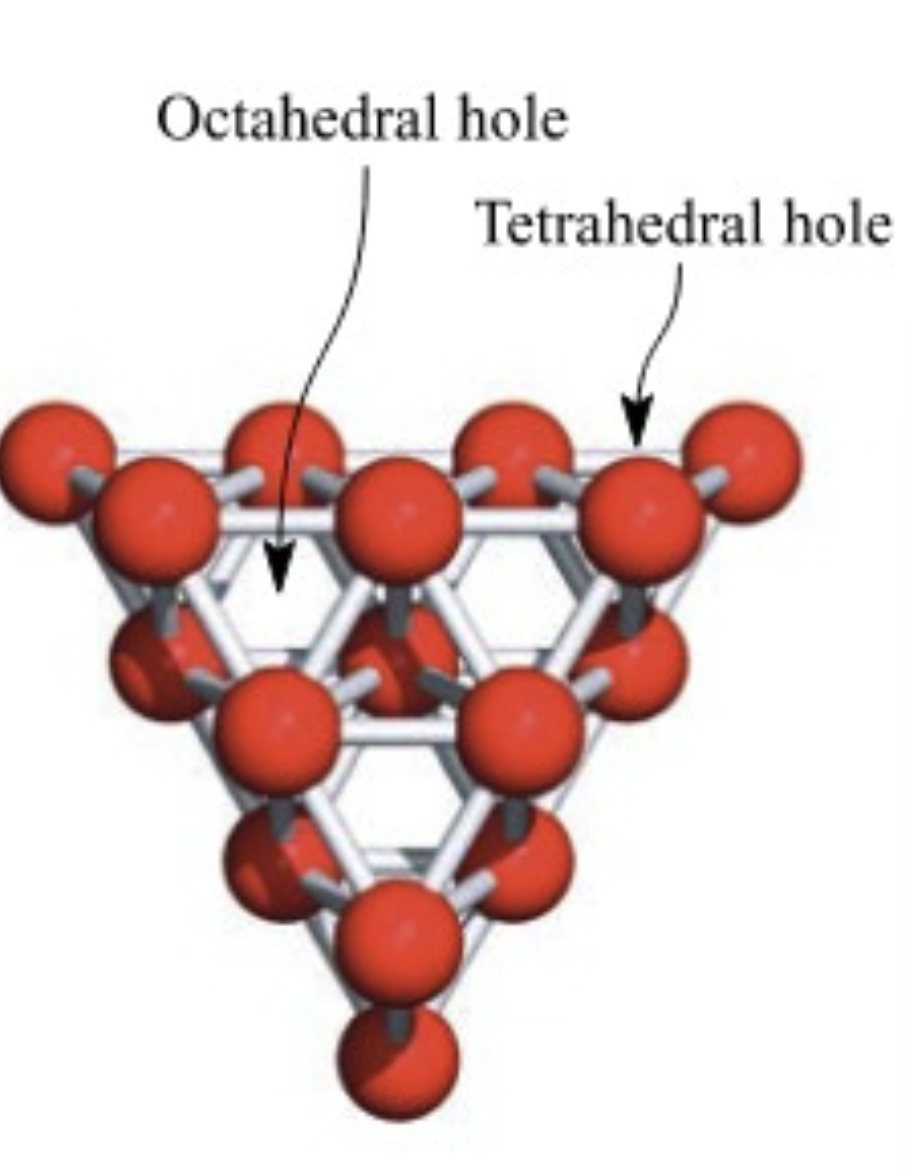
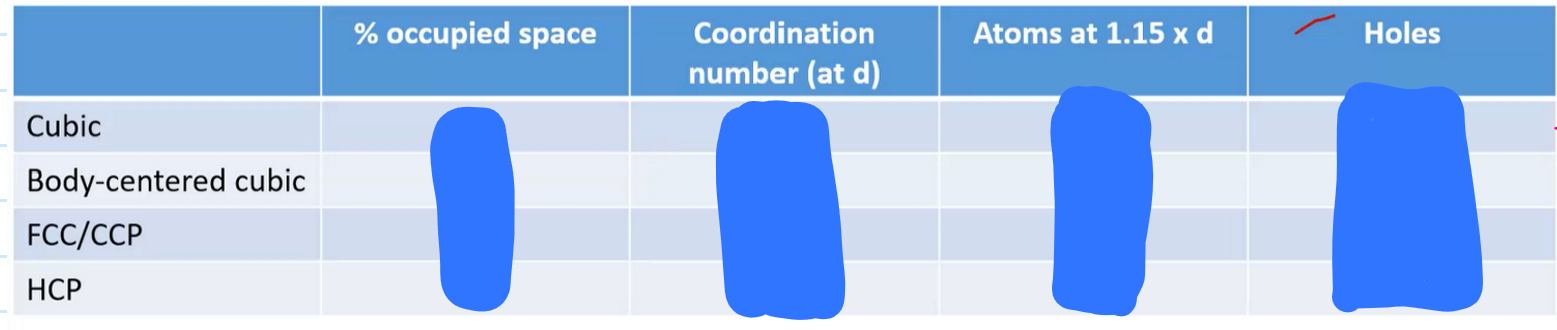
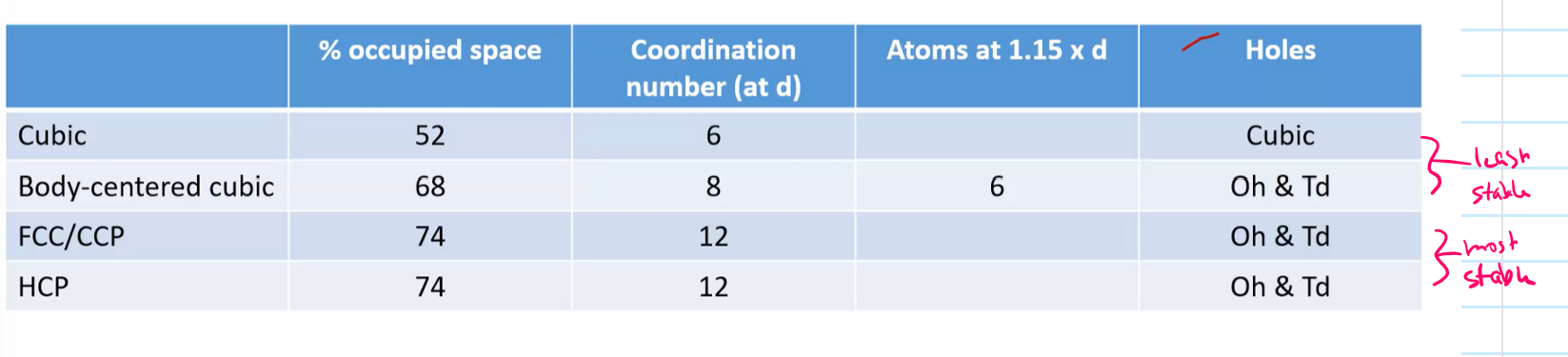
What type of holes can close packing structures have?
Octahedral
surrounded by 6 spheres
Tetrahedral
surrounded by 4 spheres
Simple cube
primitive cube
Basic
Efficiency is 52%
6 neighbors
Coordinate number = 6

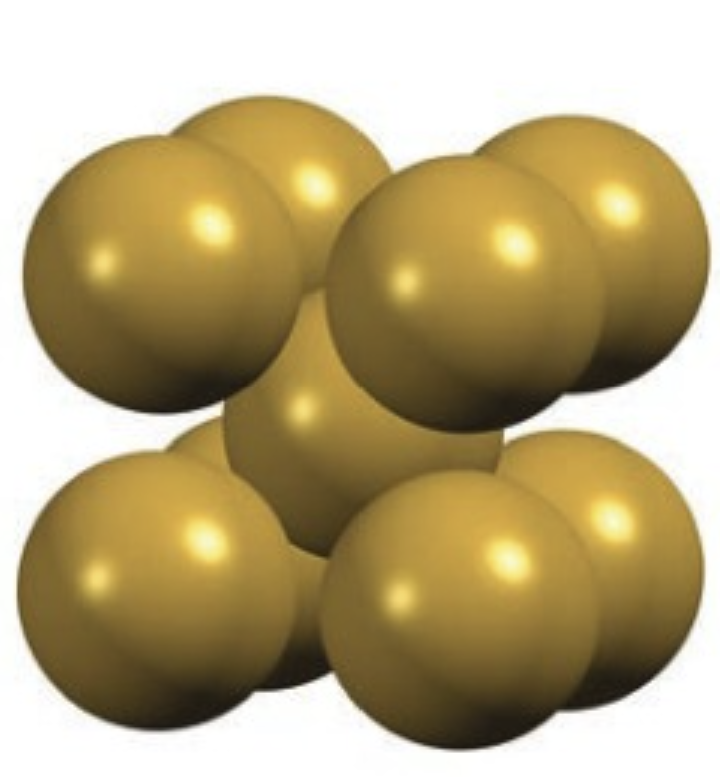
Body-centered cubic
bcc
Sphere in the middle
Efficiency is 68%
Coordinate number = 8
1 sphere in the middle, and outer corners spheres = 1 leading to 2 complete spheres total inside
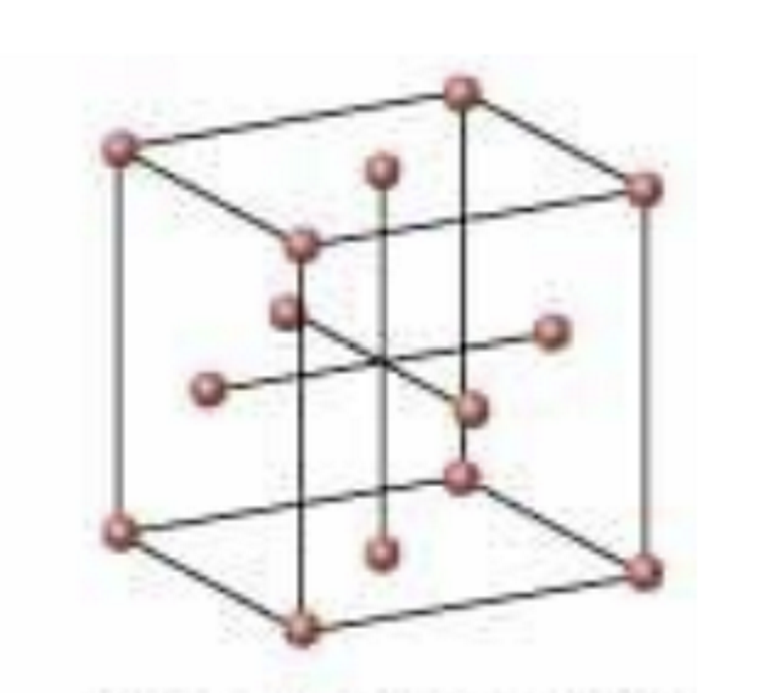
Face-centered cubic
74% efficiency
Coordinate number = 12
Each of the 8 spheres add up to 4 complete spheres inside
Number of spheres per unit cell?
8 × 1/8 = 1
Alloys
A mixture or compound with 2 or more metals or non metals, alloying it with resistance to corrosion or heat.
stainless steel is an alloy steel
Resistance
resistivity x length of wire/ cross section of wire
inverse of this is electrical conductivity
metals : as temperature increases, resistance increases, conductivity decreases
semi conductor : as temperature increases, resistance decreases, conductivity increases
As temp increases
Conductivity decreases
Band
A group of MOs where the energy differences between are so small that the system behaves as if continous
farther a part the bands are the less of a conductor it is
Fully touching is conductor
far away is an insulator
Insulator band

Metal with partially filled lower band
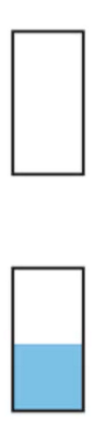
Metal with overlapping occupied and empt bands
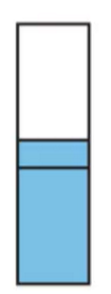
Semiconductor
Fermi level
chemical potentials of electrons in a solid (conductor, semi conductor, insulator
Intrinsic semiconductor
no dopants
small band gap
Completely filled and completely empty bands
Conductivity increases with temperature
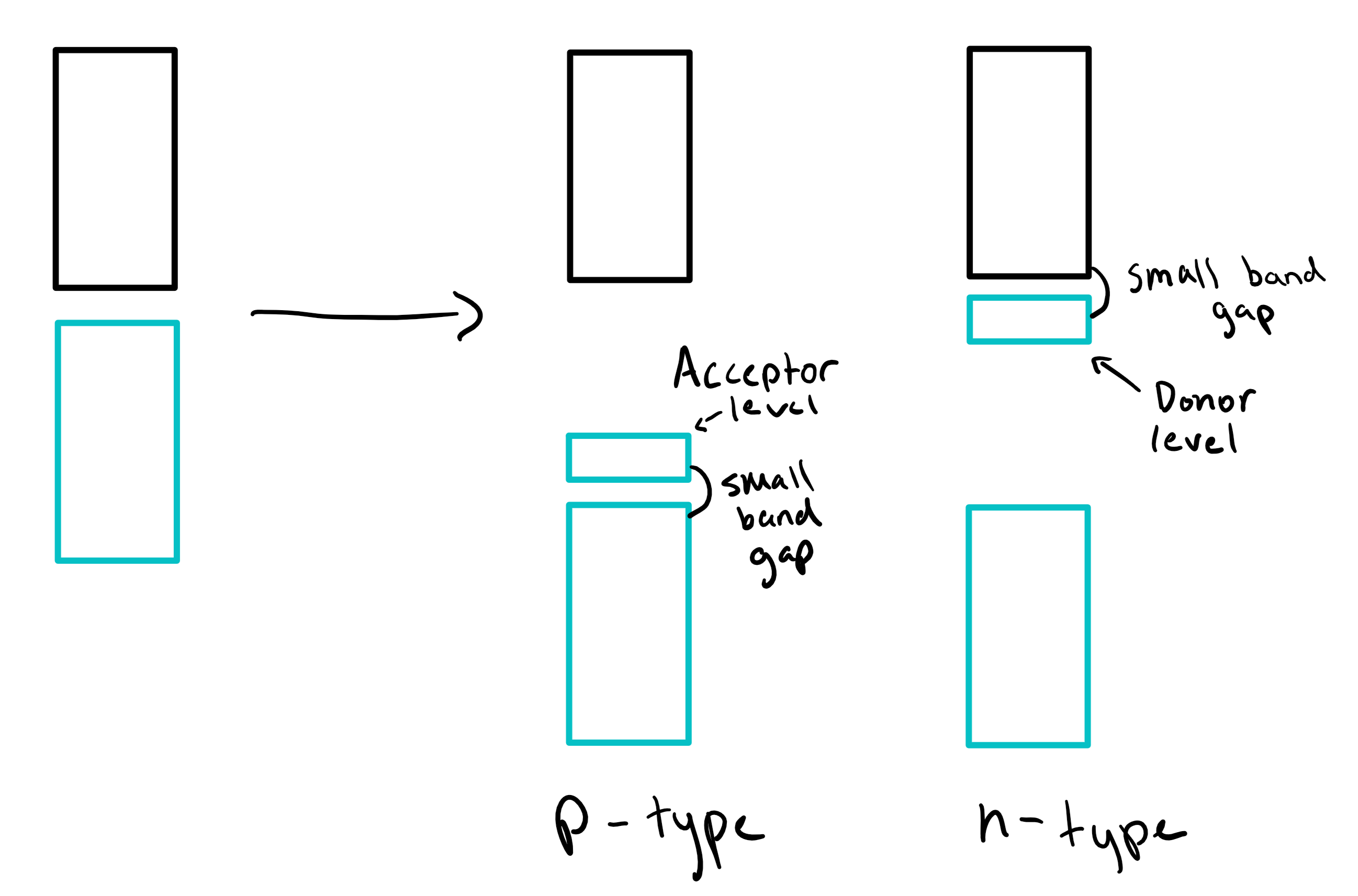
Extrinsic semiconductor
contains dopants (a small amount of an element) with more n-type or less p-type than 4 e-
Reduction Oxidation reaction
Oxidation = losing one or more electrons
Reduction = gaining one or more electrons
standard Gibbs free energy change
ΔG° = - nFE°cell
n= how many e- transferred
Faraday’s constant = 96485
E°cell = standard cell potential in volts
Cathode vs anode
To find E°cell
Cathode (reduction E°cell) - Anode (oxidation E°cell)
remember that if it is oxidation the E°cell is going to be positive
To write a balanced spontaneous cell equation
Write the two separate equation as a oxidation and reduction equations
Reduction = cathode
Oxidation = anode
E cell = +
Than combine for the balanced equation
To find E cell = cathode - anode
If positive than it is sponateous
To find Delta G
Delta G = -(how many e- transferred) (Faradays constant)(E cell)
Lattice energy
delta U
change in internal energy from gas to solid