HRM - Lecture 9: Role of Kidney in Acid-Base Balance
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
- Buffers in blood and tissues bind H+
- Lungs: eliminating CO2 and thereby indirectly H+
- Kidneys: Slow but powerful. Eliminates non-volatile acids
What are the 3 main pH regulating mechanisms of body?
- proteins
- phosphate
- ammonia
- bicarbonate
Name 4 general buffer molecules
- H+ secretion
- Bicarbonate reabsorption
- Production of new Bicarbonate
By which 3 actions does the kidney remove H+ from the body?
Normally, urine will be acidic as the body produces many metabolic acids which must be excreted in the urine
Though urine pH has a range of normal values, would you expect it to generally be acidic or basic in a normal person? Why?
- Normally, bicarbonate excretion is close to zero
- If bicarbonate were not to be reabsorbed, loss of this essential buffer would lead to acidification of the body
What is the rate of bicarbonate excretion under normal conditions? What would happen if it increased?
PCT: 80-90%
Where is most bicarbonate reabsorbed? (which segment of the nephron)
Normal: 4.5-6.6 (average is 6)
Maximal: 4.5-8
What are the normal and maximal urine pH range respectively?
Hours to days (3-6days)
How long does the kidney take to respond to pH changes?
Secondary active transport via NHE: sodium hydrogen exchanger in luminal membrane
By which transport mechanism is H+ secreted?
- Decreased H+ secretion
- Decreased Na+ reabsorption -> diuresis
What is the effect of carbonic anhydrase inhibitors at the kidney
TAL (10%)
CD: (4.9%)
What are the other locations of H+ secretion, aside from the PCT?
- First, bicarbonate must combine with a H+ to form carbonic acid in the lumen.
- H2CO3 then dissociates into H20 and CO2.
- H20 and CO2 can diffuse into the luminal cells
- In the luminal cell, H2O and CO2 recombine to make H2CO3
- H2CO3 dissociates into H+ and HCO3-
- H+ is exchanged for an Na+ at the lumen and HCO3- is reabsorbed at the basolateral memrbane via cotransport with Na+
How is bicarbonate reabsorbed?
Angiotensin II and Norepinephrine
Which hormones stimulate Na+/H+ exchange?
Only a small amount; the pH goes from ~7.4-7
How much acidification is achieved in the PCT via NHE?
late distal convoluted tubule and collecting duct
Which sites are responsible for the remainder of H+ secretion after the PCT (NET acid secretion)?
Type A intercalated cells
Which cells are active in secreting H+ in the late DCT and CD?
- H+ ATPase in the luminal/apical membrane
- H+/K+ ATPase in the luminal membrane
What are the transporters/channels responsible for secreting H+
HCO3-/Cl- antiporter in the basolateral membrane
How is bicarbonate transported into blood from Type A intercalated cells?
Type B/beta intercalated cell
Which cell type is depicted?
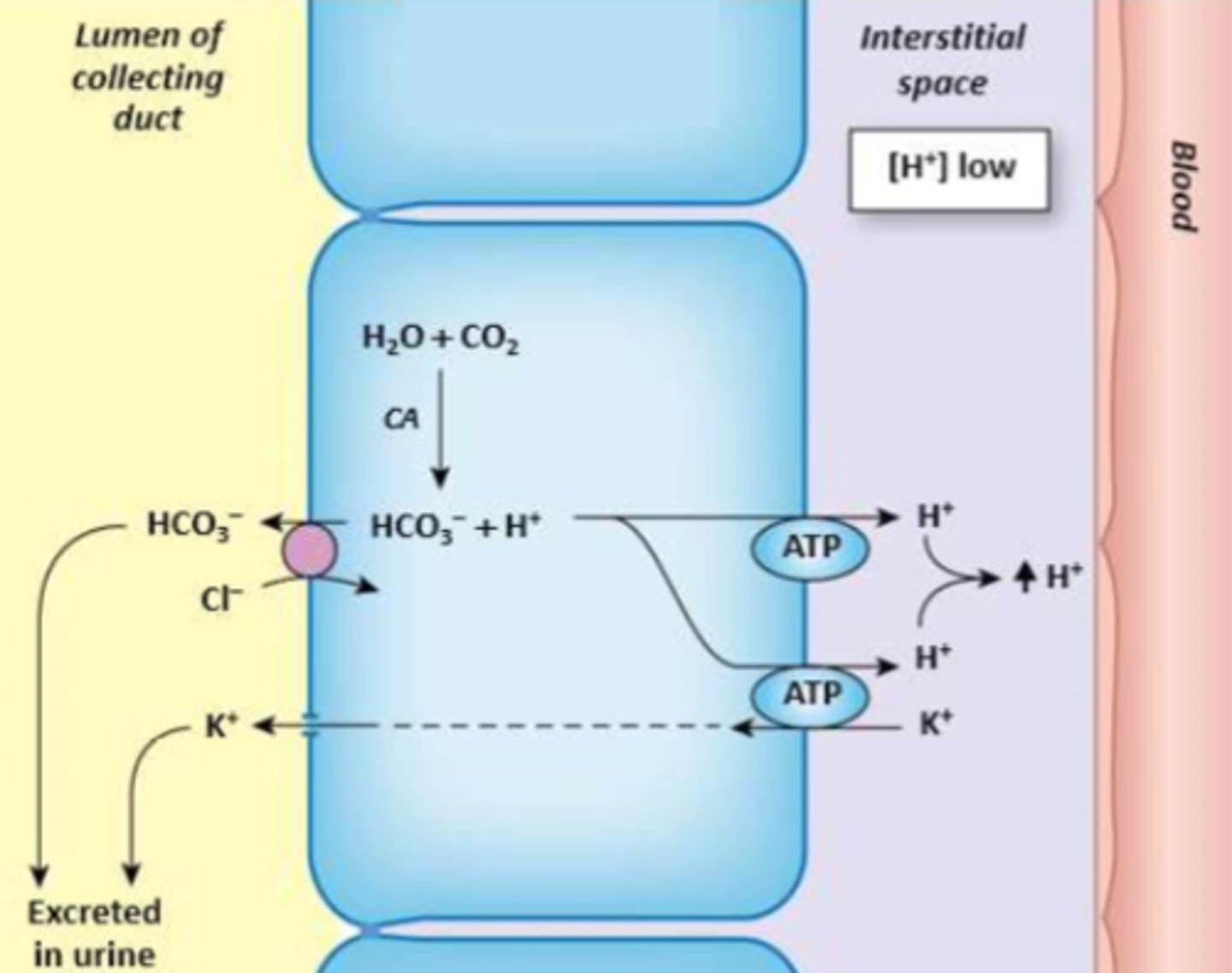
Type A/alpha intercalated cell
Which cell type is depicted?
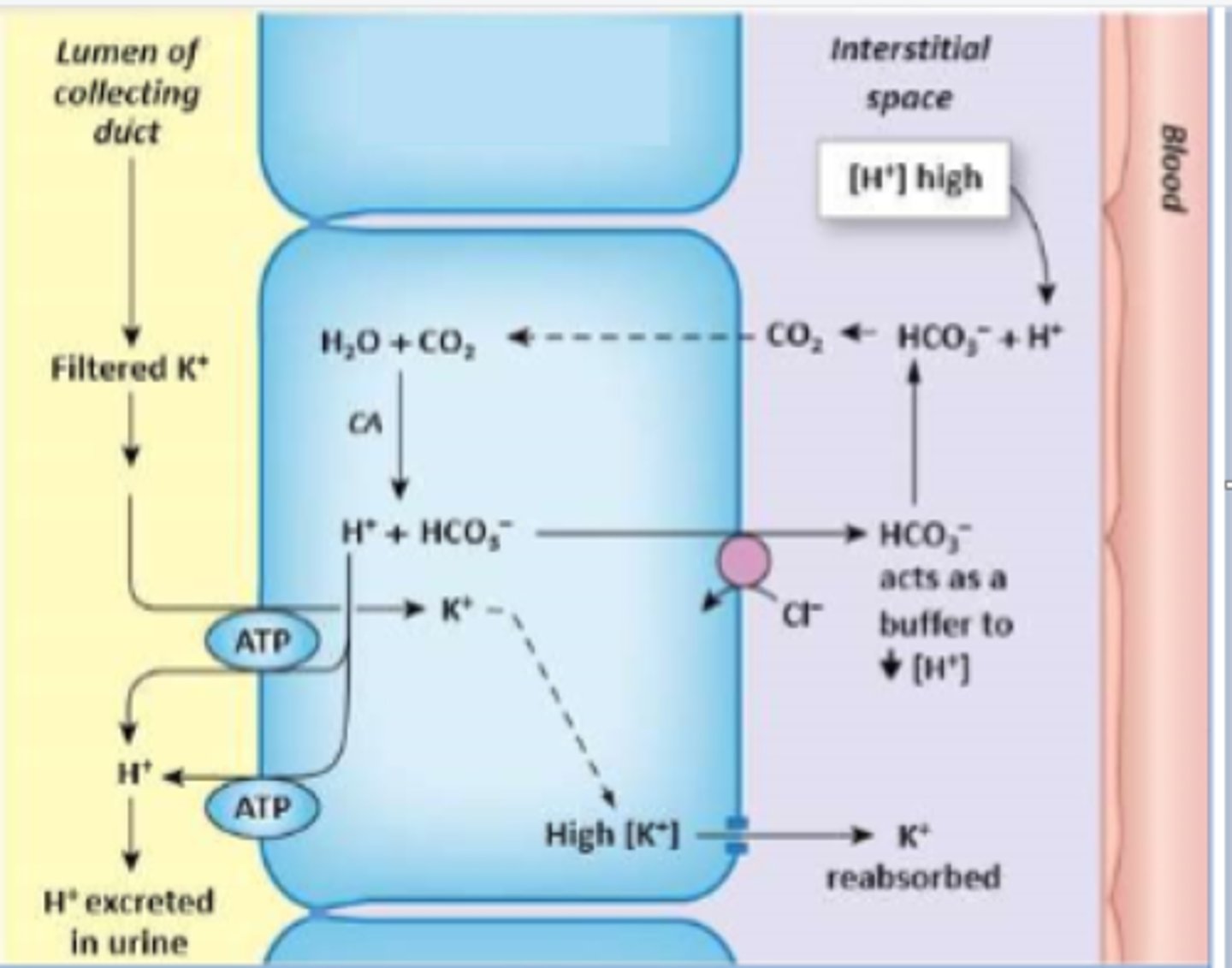
Acidosis - alpha
Alkalosis - beta
Which type of intercalated cell is active during acidosis and alkalosis, respectively?
Type B intercalated cells have the same transporters as type A, only their position in luminal/basolateral membrane is reversed.
Luminal: HCO3-/Cl- exchanger
Basolateral: H+ ATPase; H+/K+ ATPase
How do beta intercalated cells regulate bicarbonate, hydrogen, and potassium?
A pH of 4.5 limits H+ secretion due to:
- Binding with luminal HCO3-
- Binding with NaHPO4- to form NaH2PO4
- Binding with NH3 secreted by the tubule cells. This results in formation of ammonium salts
What is meant by "self-limiting" H+ handling?
This can occur in PCT, late DCT and CD. It is dependent on whether HCO3- originates from filtrate or is created via either:
- CO2 addition into alpha intercalated cell -> carbonic acid production -> dissociation into HCO3- and H+ -> H+ secretion and HCO3- reabsorption
- glutamine breakdown and H+ secretion and HCO3- reabsorption
Where is HCO3- created? (as opposed to "reabsorbed")
HPO4 2⁻ + H⁺ → H2PO4⁻
Which reaction occurs during phosphate buffering?
NH3 + H⁺ → NH4⁺
Which reaction occurs during ammonia burffering?
Glutamine
What is the most common source of ammonia in the lumen?
The PCT cells have the most glutaminase
Where is glutamine broken down most in the nephron?
DCT and CD
Where does most phosphate buffering occur?
30-40mmol/day
How much acid can be buffered by phosphate buffering?
40mmol/day
How much acid can be buffered by ammonia?
2HCO3- + 2NH4+
What does 1 glutamine yield when broken down?
Na+/NH4+ antiporter
How is NH4+ usually transported into/out of cells?
Secreted: PCT and Medullary CD
Reabsorbed: TAL
Where is ammonia absorbed and excreted?
The body is able to produce large amounts of NH3 if necessary
How are ammonia levels maintained in acidosis?
late DCT and CD
Functionally, which segments of the nephron are most involved in regulating secretion and reabsorption to compensate acidosis and alkalosis?
Na+/H+ will increase, causing more H+ to be excreted.
What effect does salt depletion have on H+ handling?
H+/K+ exchange increases and more H+ is secreted into the lumen.
What effect does hypokalaemia have on H+ handling?
Increase in H+ secretion via increased Na/H exchange in PCT
What is the effect of glucocorticoids on H+ secretion?
- Increased K+ secretion
- Depletion of K+
- Increased K+ reabsorption in exchange for H+ secretion at luminal membrane
How does aldosterone produce a metabolic alkalosis?
↑H+
Which solute is primarily altered in Respiratory acidosis?
↓H+
Which solute is primarily altered in Respiratory alkalosis
↓HCO3-
Which solute is primarily altered in Metabolic acidosis
↑HCO3-
Which solute is primarily altered in Metabolic alkalosis
- decreased renal H+ secretion
- Impaired bicarbonate reabsorption
- Altered aldosterone production of response
Give 3 causes for Renal Tubular Acidosis(RTA)?
- Decreased NHE activity in PCT results in more H+/K+ exchange in CD → more potassium lost
- Defect in H+/K+ exchange in late DCT and CD results in decreased K+ reabsorption
How can hypokalaemia result from RTA?
- Decreased bicarbonate reabsorption
- large amounts of bicarbonate flow to distal segments
- High distal hydrogen secretion results in acidic urine
- Potassium secretion due to large amount of unreabsorbed solute in late DCT and DC
Describe proximal RTA
- Inability to secrete enough H+ ions to match normal input
- Type A intercalated cell defect
- Defects in basolateral H-Cl antiporter or luminal H+ATPase
Describe distal RTA (dRTA)
60-80mmol. This is 47500 times more acid than the normal H+ concentration. This highlights the importance of constant acid excretion in urine.
How much non-volatile acid is produced a day? What is the significance?
Carbonic acid
Which acid can not be excreted by the kidneys?
Acid in urine resulting from H2PO4-
What is titrable acid?
- HPO42-
- NH3
Which 2 buffers are involved in net acid secretion?