Chemistry 1.3 -Bonding
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
How can the octet rule be expanded?
Certain elements in period 3 and below can form compounds where they have more than 8 electrons in their valence shell, because they have d orbitals that can hold 10 electrons
This tends to be silicon, phosphorus, sulphur and chlorine at A-level
Boron and beryllium also disobey the octet rule as they can form electron deficient compounds, having less than 8 electrons in their valence shell
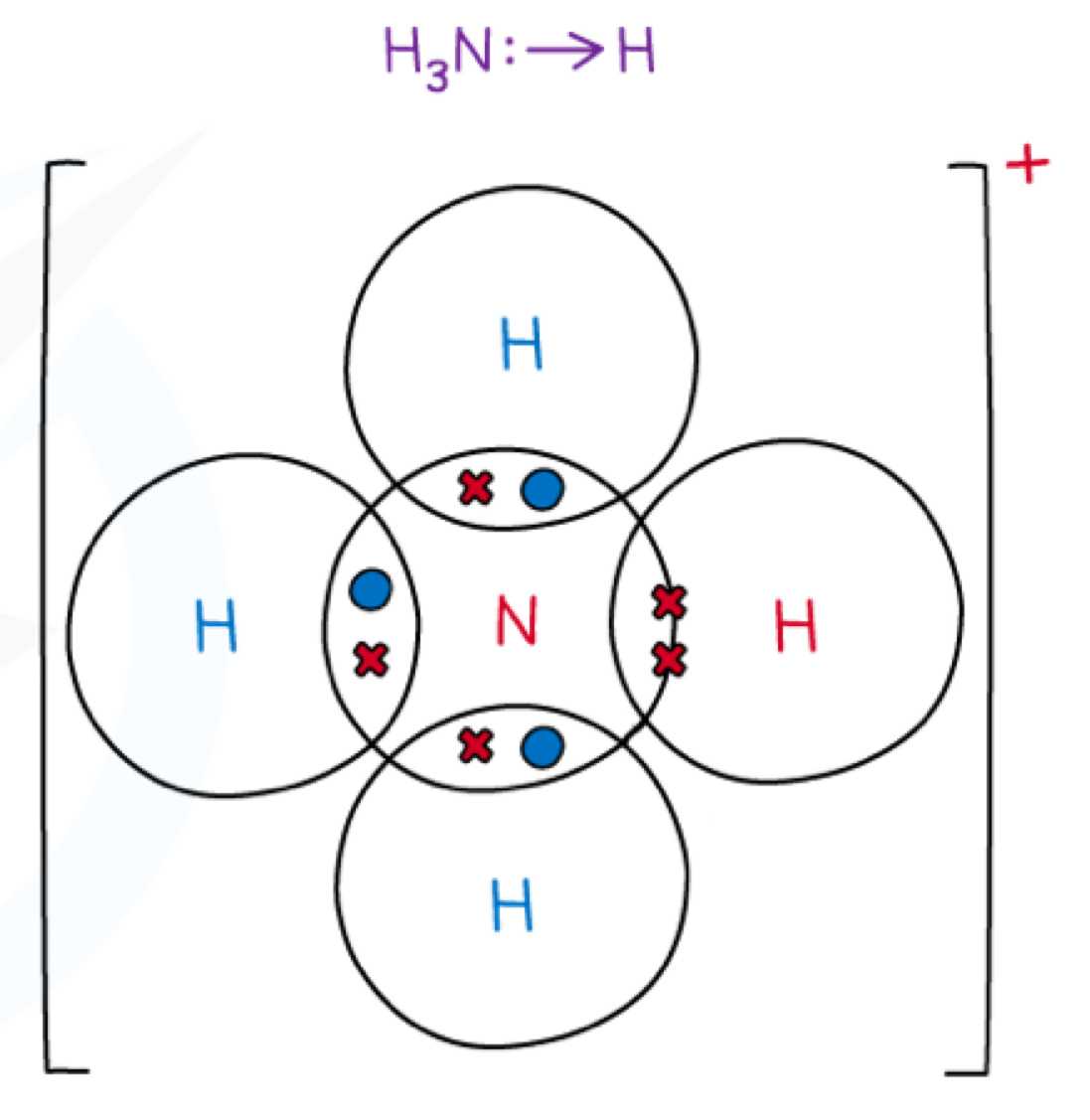
What is dative covalent bonding?
Elements with a lone pair of electrons can donate them so that both electrons within a covalent bond are from that one atom
Eg. NH4+
What rules do we use to determine the shapes of molecules?
VSEPR rules:
Valence shell electron pairs repel each other as they have the same charge
Lone pair electrons repel each other more than bonded pairs
Repulsion between multiple and single bonds is treated the same as for repulsion between single bonds
Repulsion between pairs of double bonds is greater
A molecule adopts the most stable shape to minimise the repulsion forces between pairs of electrons
So lone-lone pair repulsion > lone-bond pair repulsion > bond-bond pair repulsion
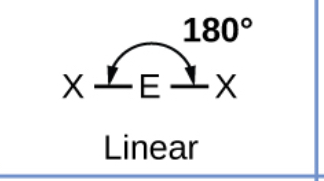
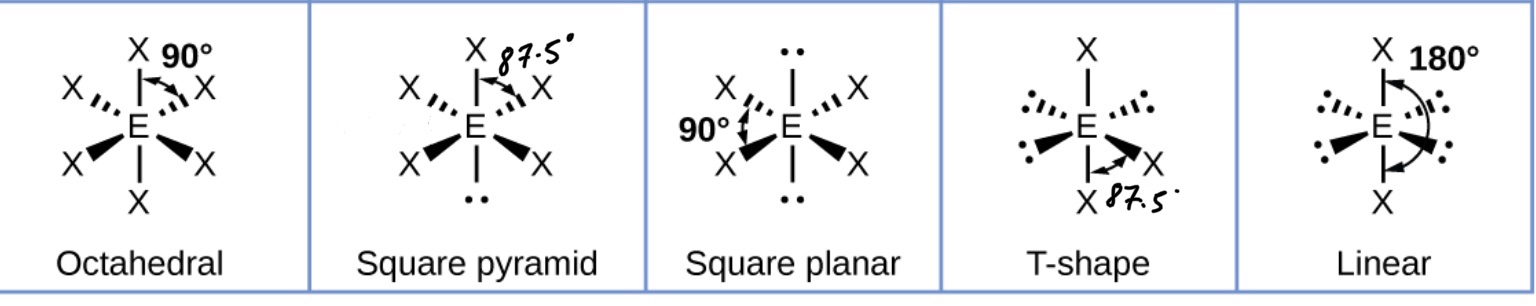
What shape will a molecule with two bonding pairs and no lone pairs take? (Include bond angles)
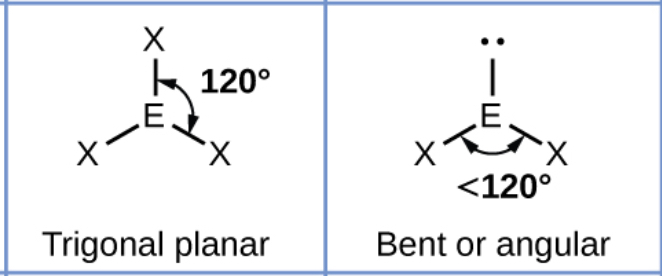
What shape will a molecule with three bonding pairs take? (Include bond angles)
What happens to that shape when you replace a bonding pair with a lone pair?
Adding a lone pair decreases the bond angles by about 2.5*
What shape will a molecule with four bonding pairs take? (Include bond angles)
What happens to that shape when you replace bonding pairs with lone pairs?
Adding a lone pair decreases the bond angles by about 2.5*
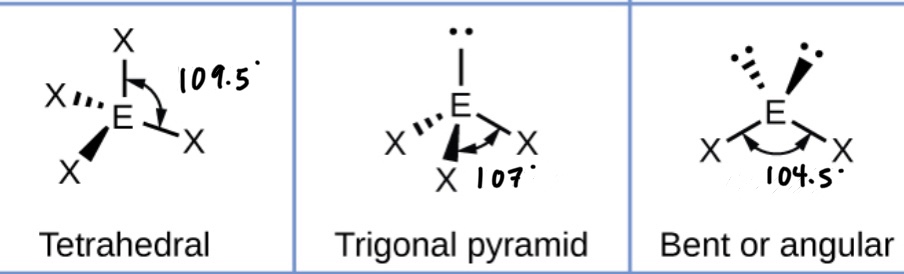
What shape will a molecule with five bonding pairs take? (Include bond angles)
What happens to that shape when you replace bonding pairs with lone pairs?
Adding a lone pair decreases the bond angles by about 2.5*
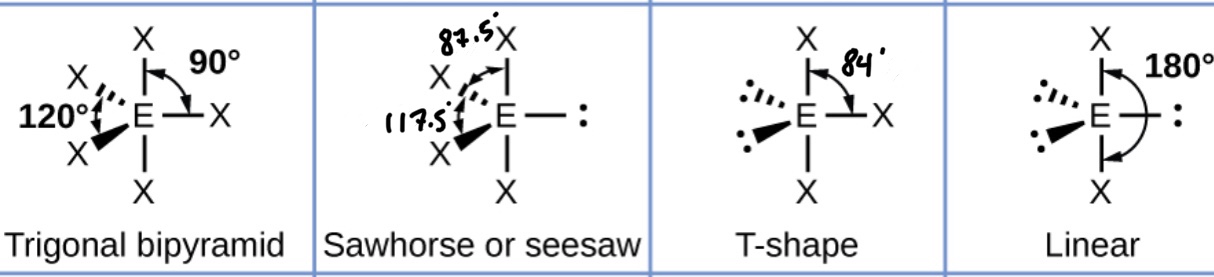
What shape will a molecule with six bonding pairs take? (Include bond angles)
What happens to that shape when you replace bonding pairs with lone pairs?
Adding a lone pair decreases the bond angles by about 2.5*
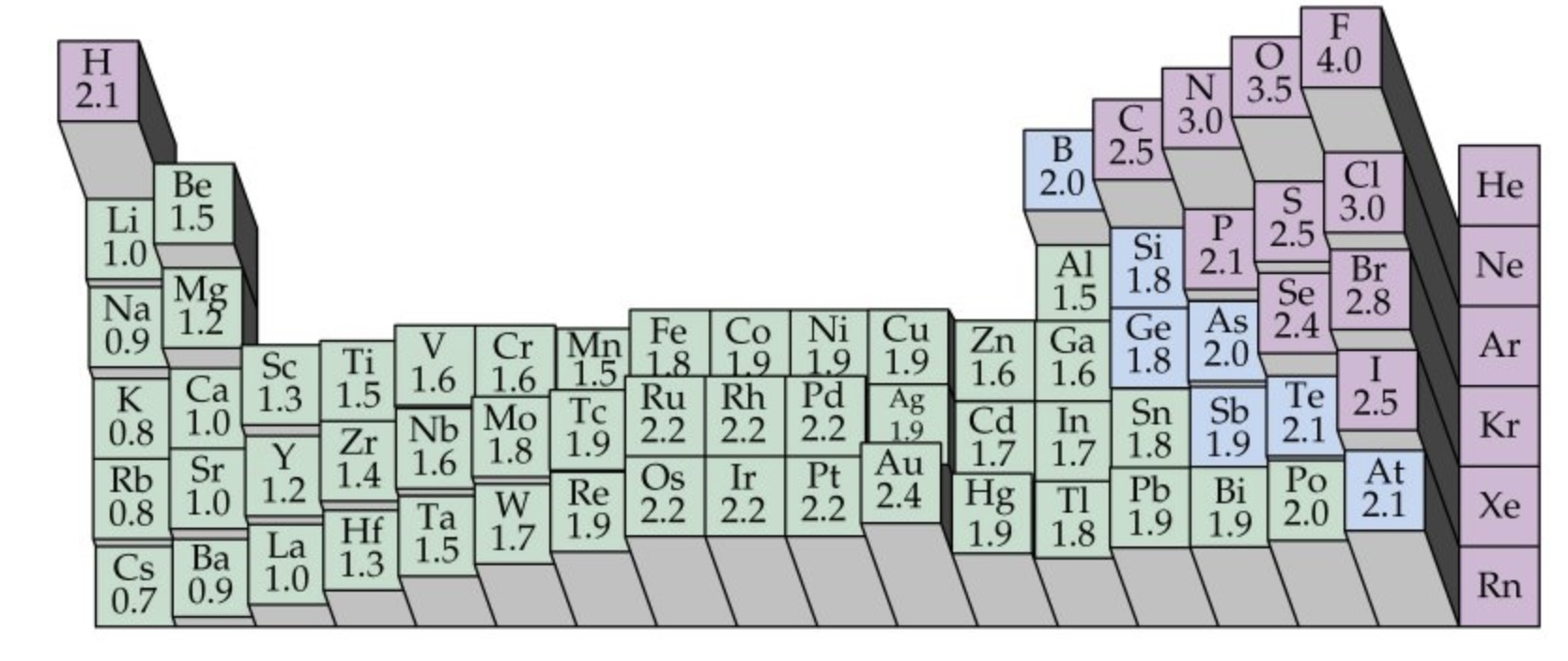
What is electronegativity?
What does this mean for bonds between different elements?
What is the scale for electronegativity?
Electronegativity is the power of an atom to attract the pair of electrons in a covalent bond towards itself
A covalent bond between elements with different electronegativities will be unsymmetrical, resulting in a polar bond
Fluorine, the most electronegative element, has a value of 4.0
What factors give elements different electronegativites
Nuclear charge
The attraction between the positive nucleus and the electron shells increases with an increasing number of protons
So an increased nuclear charge results in an increased electronegativity
Atomic radius
In atoms with a large distance between the nucleus and outer shell, the outer electrons will be less strongly attracted
So an increased atomic radius results in a decreased electronegativity
Electron shielding
Filled inner shells repel the outer electron shell, so the outer electrons will be less strongly attracted
So adding more shells results in a decreased electronegativity
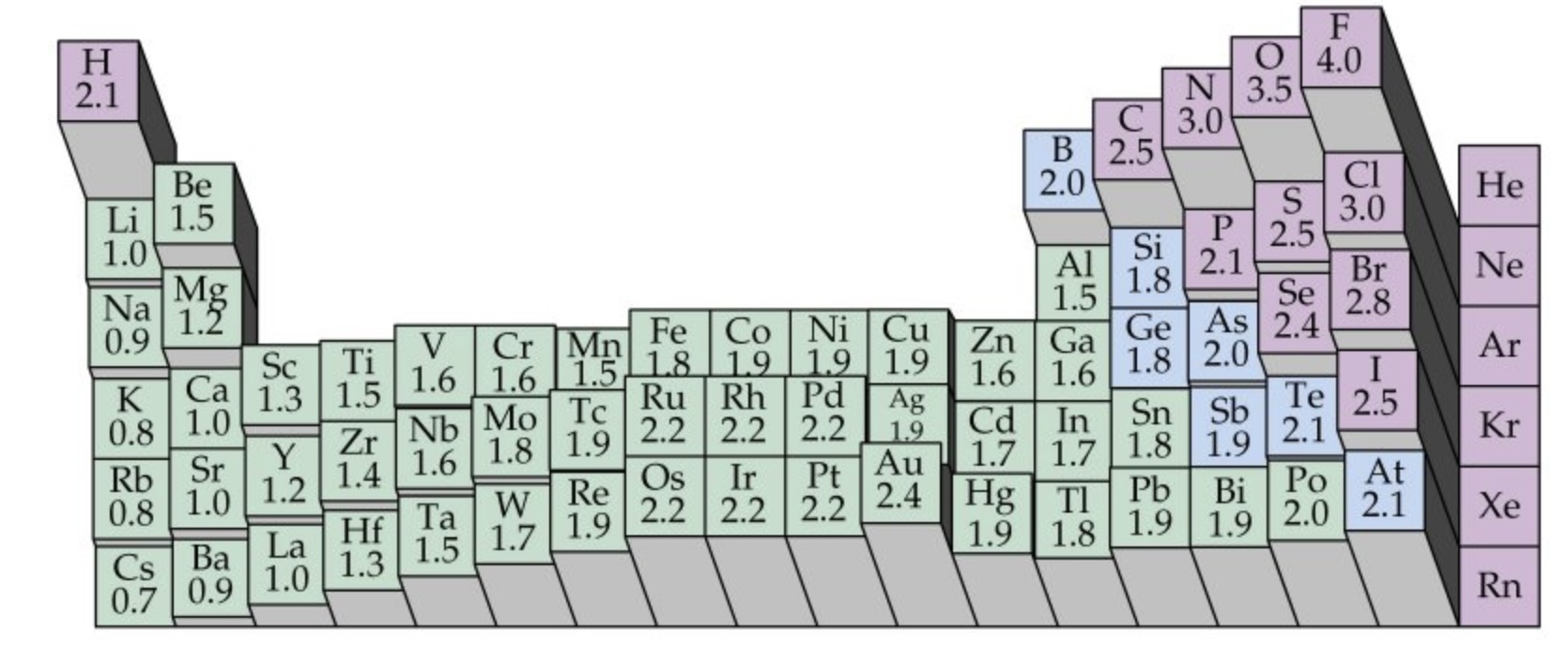
What is the trend in electronegativity down a group?
Down a group electronegativity decreases
The nuclear charge increases as more protons are added to the nucleus
But each element has an extra electron shell, which increases electron shielding
The addition of the extra shells increases the distance between the nucleus and the outer electrons so atomic radii increase
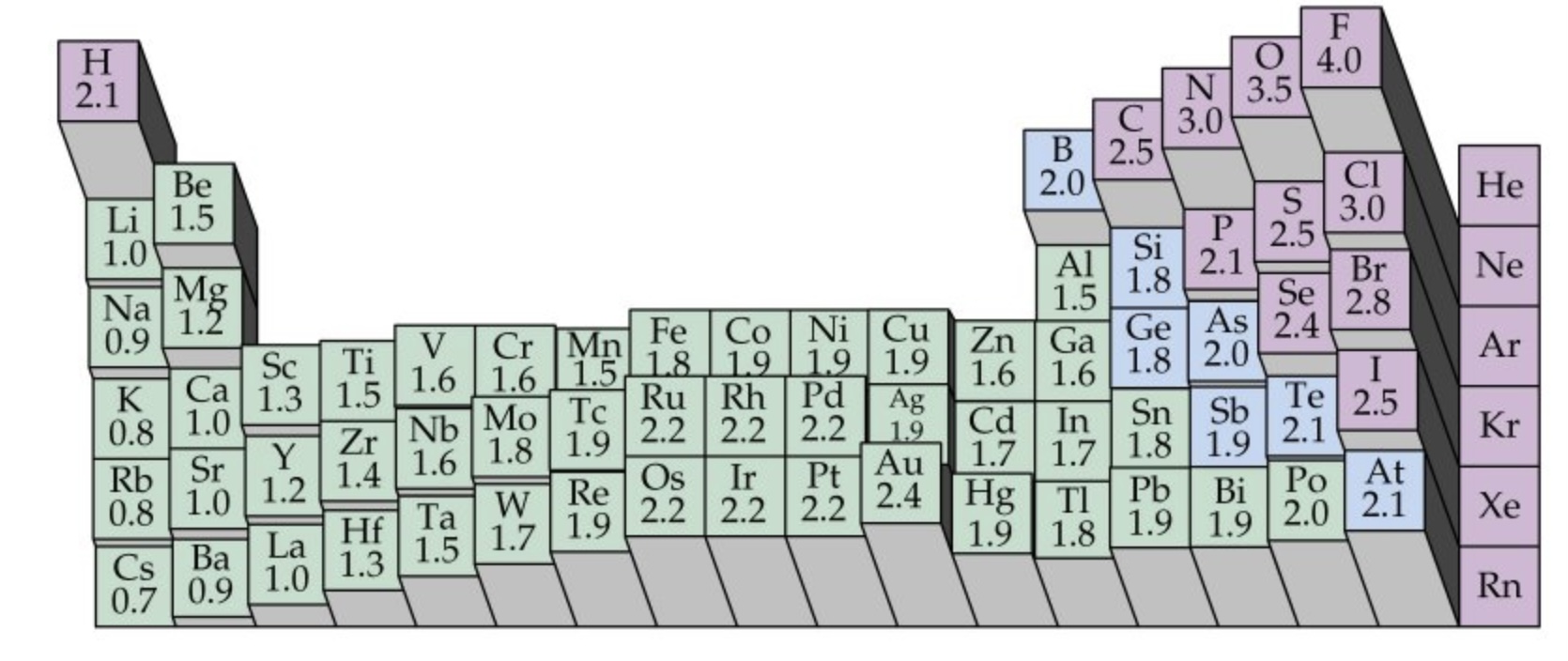
What is the trend in electronegativity across a period?
Across a period electronegativity increases
The nuclear charge increases as more protons are added to the nucleus
This pulls the electron shells in more so the atomic radii decrease
Shielding remains constant as no new shells are being added
What difference in electronegativity results in a non-polar covalent bond?
0.0-0.4
What difference in electronegativity results in a polar covalent bond?
0.4-1.8
What difference in electronegativity results in an ionic bond?
>1.8
What are the relative strengths of intra and intermolecular bonds from highest to lowest?
Strongest
Intramolecular bonds
Hydrogen bonds
Permanent dipole-dipole forces
Van der Waals (London or dispersion) forces
Weakest
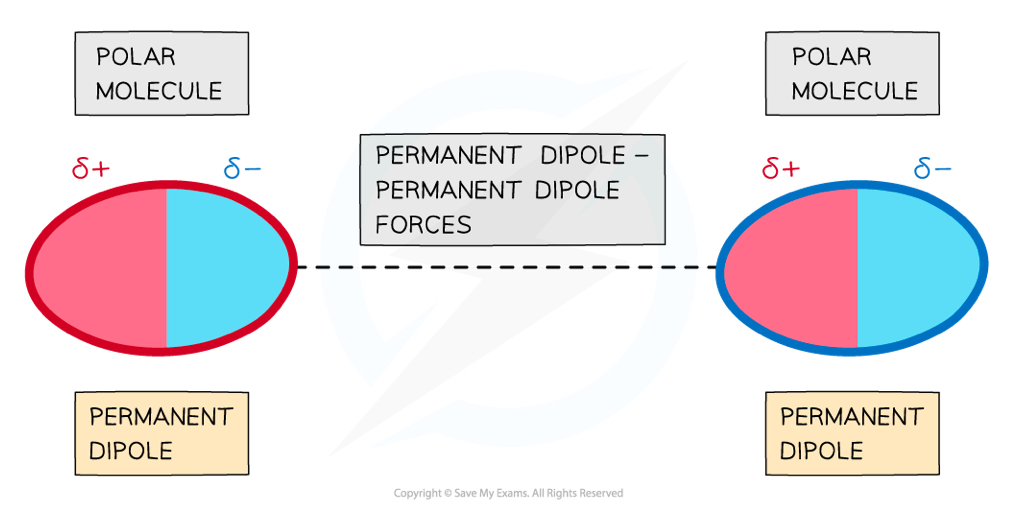
How are permanent dipole-dipole forces produced?
Polar molecules have permanent dipoles, meaning the delta positive charge from one molecule will attract the delta negative charge of another molcule
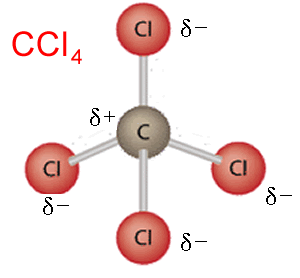
Why do some molecules with polar bonds not form permanent dipole-dipole bonds?
If a molecule has multiple polar bonds but is symmetrical, the delta charges will cancel each other out as there will be no one area of overall positive or negative charge, eg. CCl4
How are Van der Waals forces produced?
The electrons in non-polar molecules or atoms are constantly moving, and can accumulate on one side
This creates an instantaneous dipole due to an electron charge cloud
This dipole can induce a dipole on neighbouring molecules
These dipoles attract each other
Because the electron clouds are moving constantly, the dipoles are only temporary
Atoms or molecules with more electrons have stronger Van der Waals forces because the electron charge cloud is more concentrated, so they have higher melting and boiling points
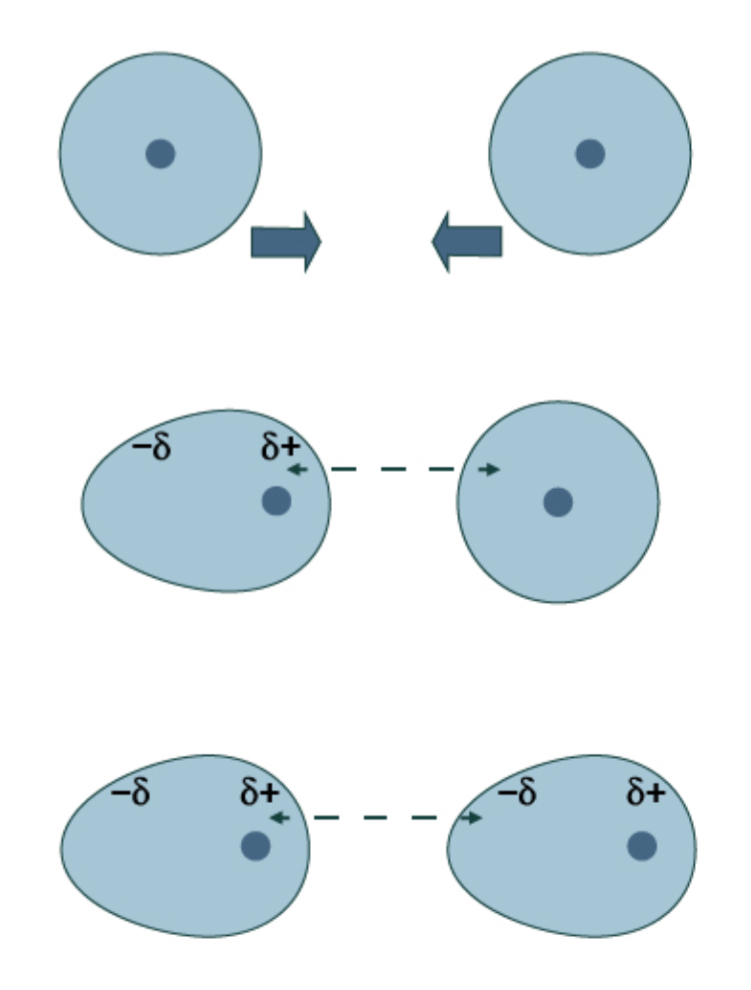
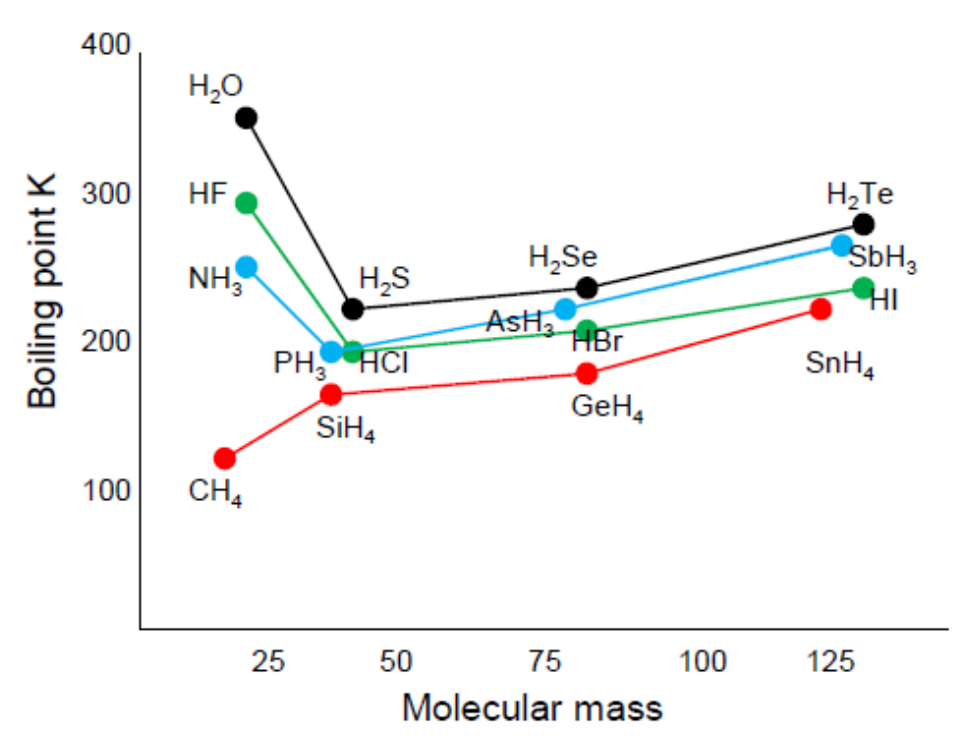
How are hydrogen bonds formed?
Bonds between hydrogen and nitrogen, oxygen or fluorine are highly polar, giving the hydrogen such a strong delta positive charge that it can bond with the lone pair of a nitrogen, oxygen or fluorine atom
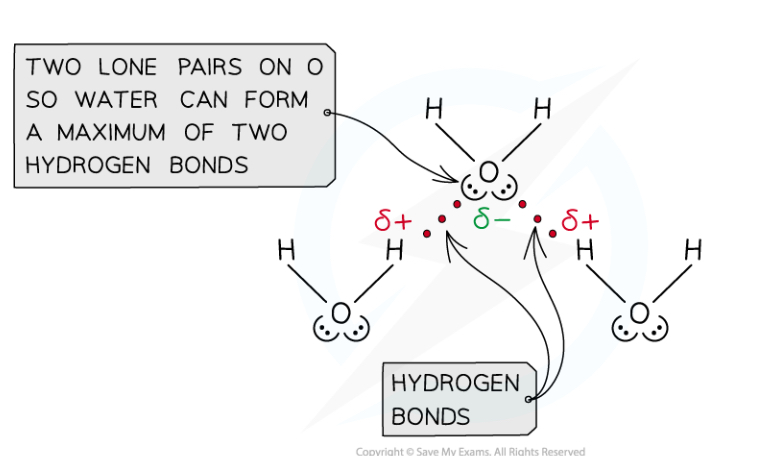
How do hydrogen bonds contribute to the properties of water and other compounds?
Compounds that can form hydrogen bonds have abnormally high melting and boiling points for simple molecules as the hydrogen bonds require more energy to overcome than Van der Waals
They also have higher surface tension as the surface molecules are attracted more by nearby molecules
Ice has a lower density than liquid water as the hydrogen bonds create a lattice structure with molecules further apart