Pharmacotherapeutics V Exam I
1/198
Earn XP
Description and Tags
Principles of Infectious Diseases, Pharmacology & Antibiotic Coverage, Antifungal Pharmacology, and Antifungal Clinical
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
199 Terms
Infection
Invasion of an organism’s body tissues by disease-causing agents, their multiplication, and the reaction of host tissues to these organisms/toxins
Caused by viruses, prions, bacteria, parasites, fungi, etc.
Transmitted via physical contact with an infected individual ot their body fluid, contaminated food or water or by touching contaminated objects
Normal flora
represents bacteria colonized in areas of the human body
patients with a history of recent antimicrobial use may have this altered
prophylactic or preventative indications
prevent initial infection or its recurrence after infection
limited to patients at high risk of developing infection
immunosuppressed patients, surgical prophylaxis, etc.
primary or secondary infection
empiric therapy indications
infecting organism(s) not yet identified
more “broad spectrum” (i.e. covers more pathogens)
based on data and public health trends → we know the most common cause of pneumonia so we can treat before we know the bacteria and then change
definitive therapy indications
organism(s) identified and specific therapy shosen
more “narrow spectrum” (i.e. covers less pathogens)
specific to the pathogen identified in the patient
colonization
the presence of organisms that are not causing disease
a.
already collected sample (start therapy to likey cover) and not not know what bacteria is causing
JJ is admitted to the hospital with a urinary tract infection. The doctor has collected a clean-catch urine sample. Which of the following type of therapy is good to initiate for JJ?
a. empiric therapy
b definitive therapy
c. prophylactic therapy
d. no therapy is needed
selecting antibiotics
3 things to think about patient, pathogen, and drug
we usually do not know the pathogen right away
it takes 2-3 days for cultures to finalize
need to treat empirically and adjust once the pathogen is identified
empiric regimens
are identified based on known information about the infection source
antibiogram
susceptibility rates in a given institution
used to guide empirical therapy
confirm the presence of infection
careful history and physical examination
signs and symptoms
predisposing factors
identify the pathogen
collect infected material
gram stain
culture and sensitivity
select empiric therapy
host factors
drug factors
monitor the therapeutic response
clinical assessment
laboratory test
assessment of therapeutic failure
presence of infection
fever
elevated WBC (greater than 10)
local signs: swelling, erythema, tenderess, purulent drainage, etc.
patient complaints: headache, chest pain, burning while urinating, etc.
avoid using antibiotics when they are not needed (i.e. self-limited conditions or viral infections)
host factors
drug allergies
age
pregnancy
genetic concerns
renal & hepatic function
concomitant drug therapy
underlying disease states
drug factors
pharmacokinetic & pharmacodynamic considerations
tissue penetration & site of infection
drug toxicity
adverse effects
cost and availability
combination antimicrobial therapy
resistance
drug allergies
confirm true allergy over adverse effects → all medications
age
determines the patient’s ability to renally eliminate the medication
neonatal concerns
pregnancy
fetal risk
altered pharmacokinetic disposition of certain medications → all the time or during certain timersters
breastfeeding
genetic or metabolic abnormalities
glucose-6-phosphate dehydrogenase deficiency
HLA-B genes
renal & hepatic functions
reduced function can lead to drug accumulation
concomitant drug therapy/underlying disease states
drug-drug interactions
trauma/burns, immunosuppression, diabetes
time-dependent killing
keep the drug concentration above the MIC for as long as possible
dosing strategies: shorter dosing interval, extended or continuous infusion
real world example: extended infusion piperacillin-tazobactam
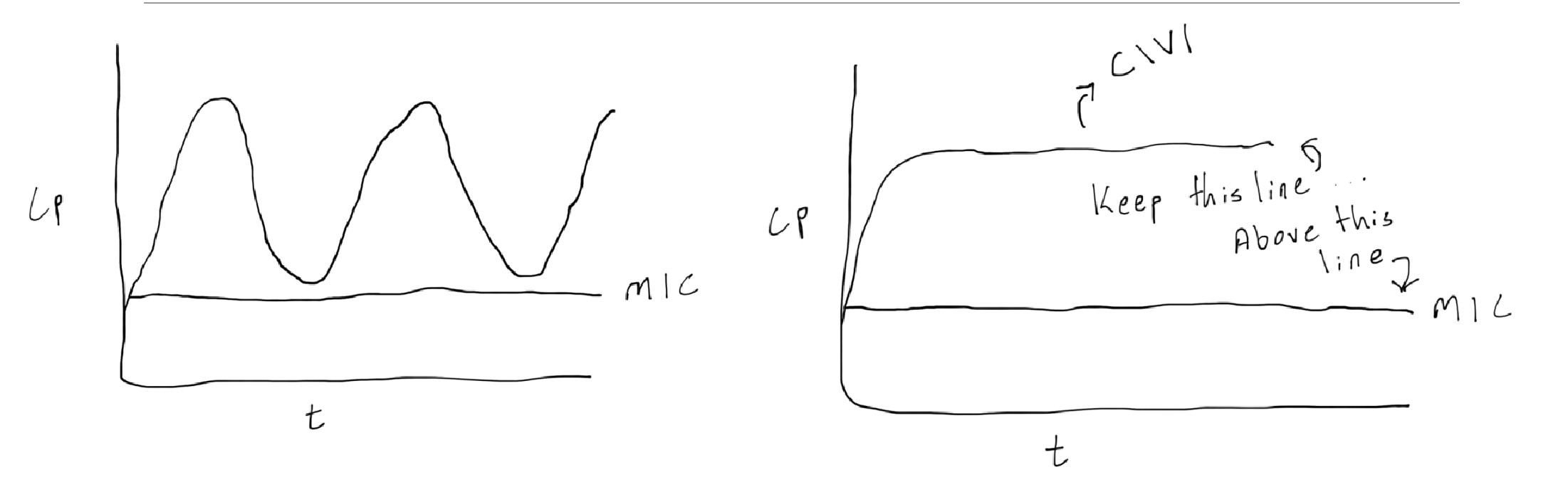
concentration dependent
achieve as high of a peak as possible while avoiding drug reactions
dosing strategies: large doses, long intervals
real world example: extended interval aminoglycoside dosing
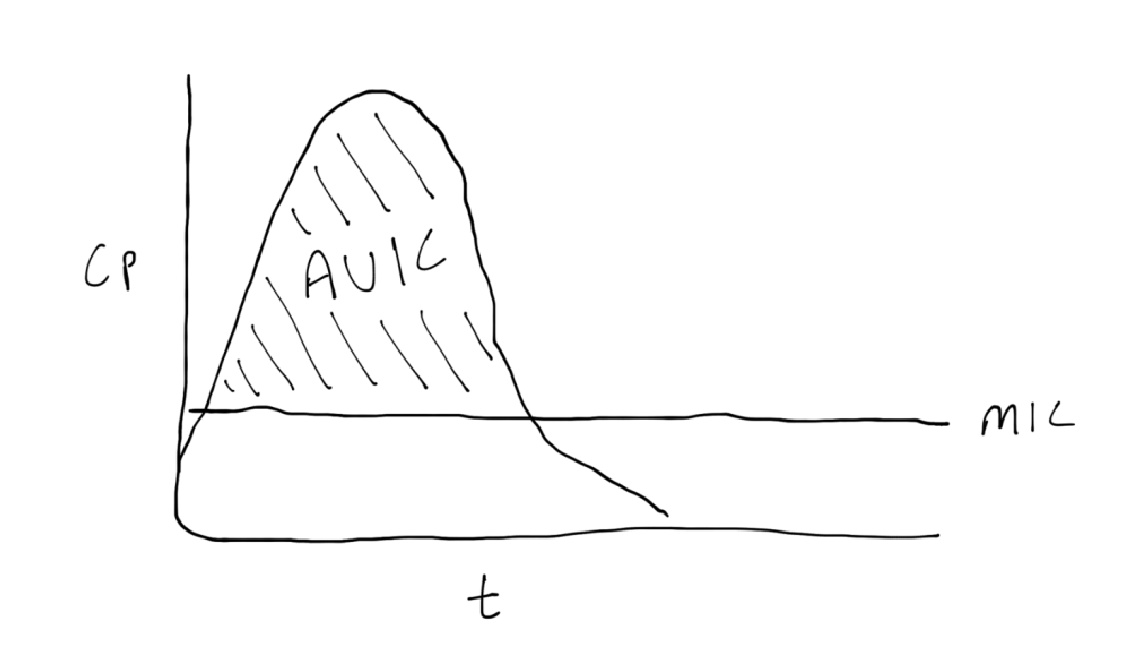
AUC:MIC
achieve maximum exposure over time
dosing strategies: variable
real world example: AUC:MIC based vancomycin dosing
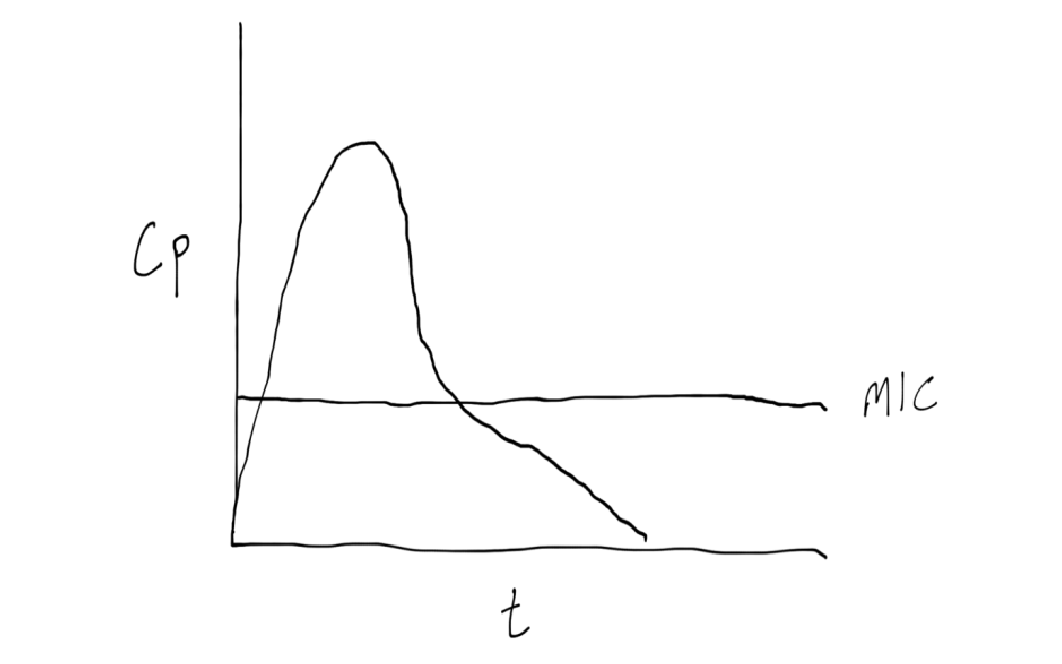
post antibiotic effect
period where the antibiotic is still killing bacteria AFTER the concentration has dropped below the MIC
real world example: extended interval aminoglycoside dosing

bactericidal
kill bacteria
tend to work on the bacterial cell wall or membrane
preferred for serious infections (i.e. endocarditis, meningitis, osteomyelitis, neutropenic infections)
bacteriostatic
inhibit bacterial growth
tend to work on bacterial ribosomes and protein synthesis
bioavailability
percentage of orally administered drug that enters the bloodstream relative to an IV formulation fot he same drug
IV=100%
food, gastric acidity, and chelating agents can also influence absorption
tissue penetration
concerning for cerebrospinal fluid (CSF), urine, synovial fluid, peritoneal fluid, and abscesses
parenteral therapy preferred for more serious infections (i.e. meningitis, endocarditis, osteomyelitis)
patients treated in the ambulatory setting generally receive oral therapy
advantages of combining antibiotics
broadens the spectrum of coverage
useful for synergy (example: beta-lactam plus aminoglycoside for endocarditis)
disadvantages of combining antibiotics
increases side effects
increased risk of resistance
increased cost
monitoring for adverse drug reactions
side effects
lab work
vitals
monitoring therapeutic response
review culture and sensitivity reports
temperature (i.e. fever resolves)
WBC trending down and normalizes
renal/hepatic function
symptom improvement
therapeutic drug monitoring → vancomycin, aminoglycosides, etc.
causes of therapeutic failure in infection
non-bacterial
causes of therapeutic failure in drug
not susceptible
does not penetrate site of infection
inadequate does
noncompliance/missed doses
drug interactions
causes of therapeutic failure in host
immunosuppression
inadequate vascular supply to site of infection
peripheral vascular disease, necrotic tissue
causes of therapeutic failure in organism
pre-existing resistance
polymicrobial pathogens
development of resistance on therapy
causes of therapeutic failure in inadequate source control
undrained abscess
biofilm on foreign body
causes of therapeutic failure in laboratory error
identification error
incorrect susceptibility results
immunodeficiency
condition where the body’s immune system is weakened, making it harder to fight off infections and other disease states (i.e. cancer)
causes of immunodeficiency
medications that suppress the immune system → transplant, steroids, cancer therapies, biologics
cancer
congenital disorders
malnutrition
diabetes
consideration of IV to PO medication
clinical improvement
afebrile (not feverish) >24 hours
WBC and other labs improving
functioning GI tract
determine full length of treatment → IV and oral should add to the total length
transition from broad spectrum to narrow spectrum
goals of antimicrobial stewardship
improve patient safety and outcomes
curb resistance
reduce adverse effects
promote cost effectiveness
interventions of antimicrobial stewardship
pharmacokinetic monitoring of aminoglycosides and vancomycin to optimize doses and minimize toxicity
rapid identification of pathogens, shortening time to start effective treatment
preauthorization of select antimicrobials
timely transition from IV to PO (usually transition is from broad to more narrow)
Prevent resistance
First dose of antibiotic kills most of the pathogens
Some pathogens can hang around for a little bit
If pathogens are left behind, they can develop resistance to the antibiotic
Why do we finish all antibiotic therapy? (even if we feel better)
antibiotics usually causes an upset stomach
can impact absorption if an empty stomach is needed
Why do we take antibiotics with food?
a.
MB is a 68 year old female with a past medical history of diabetes and hypertension who is admitted to the hospital with osteomyelitis of the left foot. The team draws blood cultures in the ED and wants to start the patient on therapy right away. What therapy should be initiated at this time?
a. empiric therapy
b. definitive therapy
c. prophylactic therapy
d. colonization therapy
e. none of the above
b.
MB is a 68 year old female with a past medical history of diabetes and hypertension who is admitted to the hospital with osteomyelitis of the left foot. The patient has been receiving ampicillin/sulbactam for 3 days. The bone culture results are listed below. What therapy should be initiated at this time?
a. empiric therapy
b. definitive therapy
c. prophylactic therapy
d. colonization therapy
e. none of the above
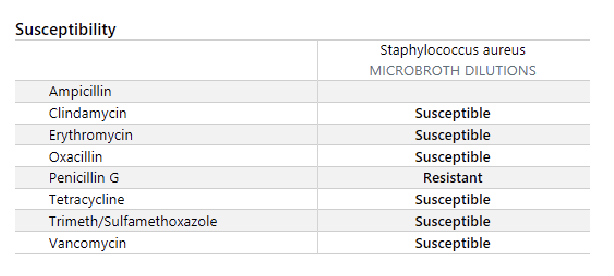
a.
Refer to the following culture report.
What sort of pathogen is Staphylococcus aureus?
a. gram positive cocci
b. gram negative rod
c. anaerobe
d. atypical pathogen

a.
b.
d.
e.
Which of the following are host (patient) factors that need to be considered when selecting antibiotics for infections? Select all that apply.
a. drug alleries
b. renal dysfunction
c. tissue penetration
d. drug internations
e. pregnancy
f. resistance
c.
JB is being treated for a urinary tract infection in the hospital and has received 3 days worth of IV Ceftriaxone. The plan is to treat the urinary tract infection for a total of 7 days. The patient is evaluated and appears to be improving. The medical team plans for discharge today. How many additional days of oral cefdinir should the patient receive to complete treatment for the urinary tract infection.
a. 2 more days
b. 3 more days
c. 4 more days
d. 5 more days
capsule and glycocalyx (polysaccharides or hyaluronic acid)
causes protection from desiccation (drying), attachment to surfaces
cell wall
peptidoglycans made of N-acetyl muramic acid and N-acetyl glucosamine crosslinked with tetrapeptides
it is porous to allow nutrients and water flow
peptide contains glutamate, alamine, and lysine
different in gram (-) and gram (+) bacteria
gram (+) is thicker causing a purple color upon staining due to stain not being able to leave
cell membrane
can be a drug target or barrier to drug penetration
gram (-) has 2
gram (+) has 1
genetic material and ribosomes
major drug target
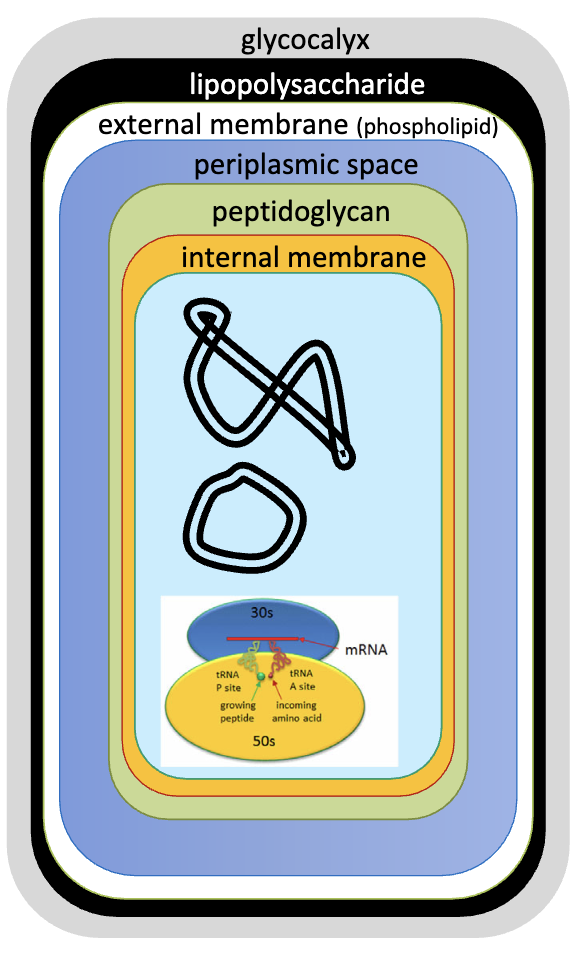
gram-negative bacteria
3 components outside the peptidoglycan layer
lipopolysaccharide (LPS): a.k.a endotoxin
external membrane (phospholipid) → second cell membrane
periplasm
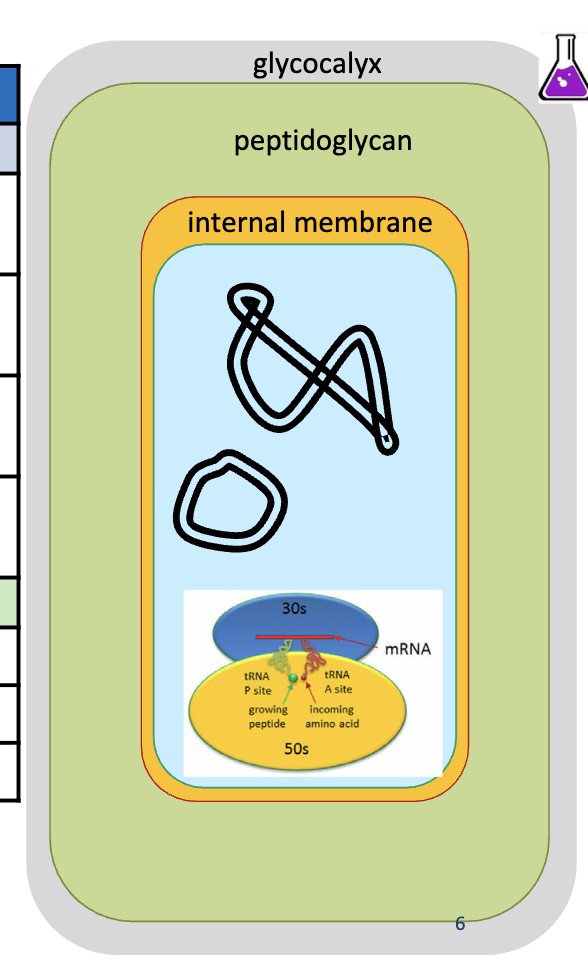
gram-positive bacteria
no lipopolysaccharide, external membrane, or periplasm
much thicker peptidoglycan layer
list of gram-negative bacteria
Enterobacteriaceae
Escherichia coli
Helicobacter
Haemophilus
Klebsiella
Legionella
Neisseria
Pseudomonas
Salmonella
Shigella
Vibrio
Brucella
Acinetobacter
list of gram-positive bacteria
Cocci
staphylococcus aureus
streptococcus pyogenes
streptococcus pneumonia
streptococcus viridans
Bacilli
Corynebacterium
Listeria
Bacillus
DNA/RNA Inhinitors
Quinolones (DNA gyrase, topoisomerase IV)
Metronidazole, tinidazole
Rifampin
Cell Membrane Inhibitors
Polymyxins
Daptomycin
Telavancin
Oritavancin
Protein Synthesis Inhibitors
Aminoglycosides
Macrolides (erythromycin, azithromycin, and clarithromycin)
Tetracyclines
Clindamycin
Linezolid, tedizolid
Quinupristin/Dalfopristin
if cells cannot produce RNA, they die
Cell Wall Inhibitors
Beta-lactams (penicillins, cephalosporins, carbapenems)
Monobactams (aztreonam)
Vancomycin, dalbavancin, telavancin, oritavancin
Folic Acid Synthesis Inhibitors (DNA Synthase Inhibitors)
Sulfonamides
Trimethoprim → often combined with sulfamethoxazole to overcome resistance
Dapsone
class of beta-lactams
penicillins
cephalosporins
carbapenems
aztreonam (Azactam) → stand alone (only one in class)
side effects of beta-lactams
Hypersensitivity reactions - ranging from mild rashes to drug fever and interstitial nephritis
central nervous system issues (confusion, dizziness, seizures at high concentration, etc.)
GI upset, diarrhea
drug interactions of beta-lactams
probenecid increases drug concentration by interfering with renal excretion
can enhance the anticoagulant effect of Warfarin by inhibiting the production of vitamin K-dependent clotting factors (except for Nafcillin and Dicloxacillin)
Most require renal dose adjustment
derivatives of β-lactam
all agents contain the β-lactam ring
spectrum of activity enhanced due to protection from β-lactamases
difference in route of administration and cost

β-lactamases
bacterial enzymes that provide antibiotic resistance by breaking the beta-lactam ring in beta-lactam antibiotics
β-lactams overall major target
to block transpeptidation
Vancomycin and derivatives major target
block terminal alanine removal, which prevents transpeptidation
Fosfomycin major target
to block MurA, which prevents transpeptidation
Cycloserine major target
to block Alr and Ddl, which prevents transpeptidation
Bacitracins major target
to block C55-PP (bactoprenol pyrophosphate), which prevents transpeptidation
β-lactams mechanism of action
prevents the cross-linking peptides from binging to the retrapeptide side-chains
inhibit the enzyme of penicillin-binding protein/PBP that crosslinks peptide chains
the cell wall starts to lyse (disintegrate) itself, destroying the bacterium (bactericidal)
however if the cell wall is already created there is nothing the antibiotic can do → used to prevent not remove
4 major β-lactam resistance mechanisms
destruction of the β-lactam by β-lactamases
modification of penicillin binding proteins (PBP) to reduce affinity of the drug for the target
impaired penetration of drug to the bacterium (a gram (-) issue)
antibiotic efflux out of the bacterial cell (a gram (-) issue)
β-lactamase inhibitors
stop bacteria from deactivating the antibiotic with their enzymes
are not administered alone, and must be given with a β-lactam drug
cannot be mixed with any random β-lactam drug, but are combined with drugs that are sensitive to the β-lactamase of targeting
EX: clavulanic acid (clavulanate) → narrow, sulbactam, tazobactam, avibactam, avibactam, and relebactam → broad and strong
Penicillin-Binding Protein (PBP)
the pharmacological target of β-lactams
the enzymes that catalyze the transpeptidation reaction
Modification:
Staphylococci has caused methicillin resistance
pneumococci and enterococci has caused penicillin resistance
impaired penetration of β-lactams
only in gram (-)
caused by the peptidoglycan layer where this class of drug work is covered by the lipopolysaccharide layer
porins are required for the class to enter the periplasmic space of the gram (-) bacteria
proins can be downregulated
antibiotic efflux
membrane proteins that actively pump antibiotics and other toxic substances out of bacterial cells
only gram (-) bacteria exhibit this resistance
pumps in the outer membrane
β-lactams absorption
highly variable from drug to drug
variability partly based on acid stability
Nafcillin is not suitable for oral use due to highly erratic absorption
Most penicillins have impaired absorption with food (except amoxicillin)
IM administration of penicillin G can cause pain, whereas IV is less bothersome
Penicillins vary in their protein binding (nafcillin > penicillin G or ampicillin), affects free drug concentration
β-lactams distribution
most distribute thoroughly into extracellular space in tissue throughout the body → do not go intracellular but can freely move throughout the body
do not accumulate in intracellular fluid owing to their hydrophilic nature
CNS penetration is poor for PCNs, except when the meninges are inflamed (bacterial meningitis)
Certain cephalosporins (cefotaxime, cefuroxime, and ceftriaxone) and aztreonam enter CSF without inflamed meninges
β-lactams excretion
most are highly renally eliminated, and dependent on renal secretion (transporters)
For PCN G, 90% of renal excretion is through tubular secretion
Nafcillin and ceftriaxone have substantial hepatic elimination compared to most
inhibitors do not affect half-life significantly
probenecid
a prescription medication primarily used to treat chronic gout and gouty arthritis by helping the body eliminate excess uric acid
used with Penicillin G (PCN G) to block its kidney excretion (OAT1 & OAT3 transporters), significantly increasing penicillin's blood levels and duration
Highly bound drugs will only exhibit rapid renal clearance if they have high affinity for renal organic anion transporters
What does plasma protein binding have to do with β-lactam elimination?
glomerulus
High binding β-lactam means poor filtration at the ______?
Only the unbound, or "free," drug can pass through the filtration barrier into the renal tubules. The glomerulus is a selective filter that is passive and acts by pressure control across capillary walls. Factors that determine what can pass through are based on size, charge, and its affinity for plasma proteins (albumin).
Why does high binding β-lactam means poor filtration at the glomerulus?
They are transported due to being lipophilic.
Despite high protein binding, these 3 drugs have short half-lives, Oxacillin, Flucloxacillin, and Dicloxacillin. Why?
Natural Penicillins
Penicillin VK (Pen VK) - PO
Penicillin G Benzathine/Penicillin G Procaine (Bicillin CR) - IM
Penicillin G Benzathine (Bicillin LA) - IM
Aqueous Penicillin G -IV
Pencilln VK
Pen VK generic name
Pen VK
Penicillin VK brand name
PO Natural Penicillins
Penicillin VK (Pen VK)
Bicillin CR
Penicillin G Benzathine/Penicillin G Procaine brand name
Penicillin G Benzathine/Penicillin G Procaine
Bicillin CR generic name
Bicillin LA
Penicillin G Benzathine brand name
Penicillin G Benzathine
Bicillin LA generic name
IM Natural Penicillins
Penicillin G Benzathine/Penicillin G Procaine (Bicillin CR)
Penicillin G Benzathine (Bicillin LA)
IV Natural Penicillins
Aqueous Penicillin G