To show the reactions of ethanoic acid with ethanol
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
8 Terms
Theory
• Ethanoic acid (CH3COOH) is a weak acid but shows typical properties of acids
• It is reacted with (sodium carbonate, magnesium and) ethanol to show this
Note: These reactions test for carboxylic acids/distinguish carboxylic acids from another substance
Procedure
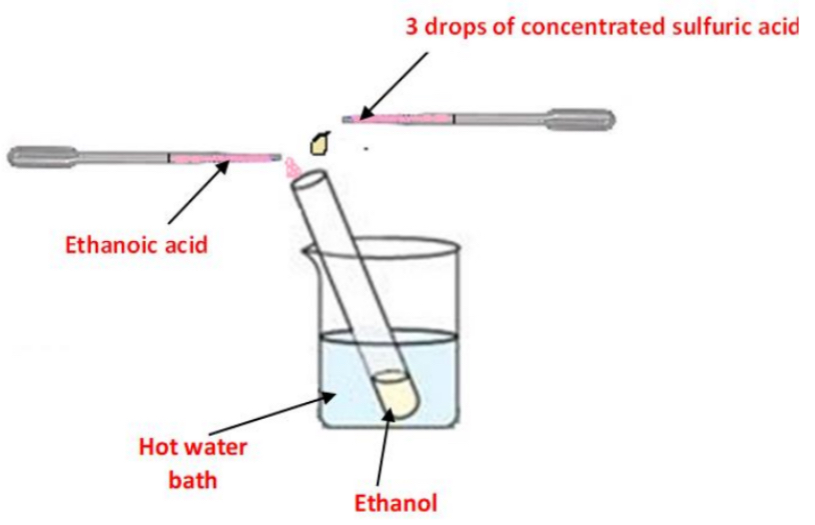
➢ A few cm3 of ethanol are placed in a test tube
➢ Using a dropper a cm3 of ethanoic acid and three drops of sulfuric acid are added and the test tube is placed in a hot water bath for about ten minutes
Result: A “fruity smell” is observed
Note: No colour change is observed
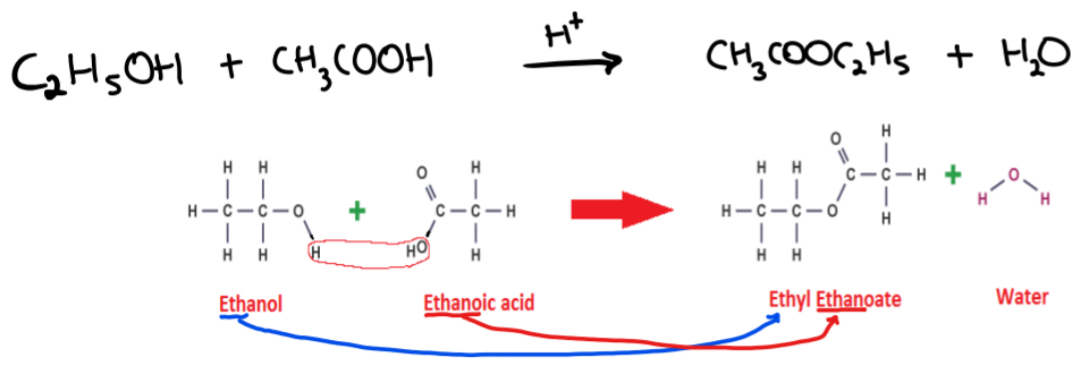
Write an equation to show the reaction of ethanoic acid with ethanol
C2H5OH + CH3COOH —H+→ CH3COOC2H5 + H2O
What is the function of the concentrated sulfuric acid?
Sulfuric acid acts as a catalyst in this reaction
Name the organic product formed
Ethyl ethanoate -(CH3COOC2H5)
To what group of compounds does the organic product of this reaction belong and how is it
identified?
Ethyl ethanoate is an ester, esters have a distinctive “fruity smell”
Why is no colour change observed in this reaction?
All of the reactants and products are colourless in this reaction
What is this type of reaction known as?
Esterification reaction (Type of condensation reaction)