Biomolecular Target Design
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
What is target oriented drug design
lead structure can be modified so that binding interactions between a drug and its target are improved
what will stronger binding lead to
greater activity
increase selectivity - stronger binding to a certain target will improve the selectivity between different targets
what do we need to remember about alkyl groups
alkyl groups bond to carbon atoms cannot be easily removed and replaced. A modified full synthesis may be required
what can varying the size and type of alkyl substituents do
can be used to probe the size and shape of hydrophobic regions in a target
what can enlargement of an alkyl substituent lead to
stronger binding to such a region
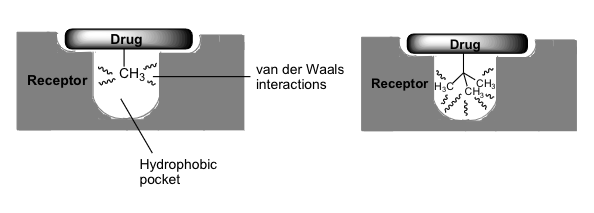
size relating to selectivity of a drug
overall size may also improve the selectivity of a drug
Aromatic substituents
relatively easy to vary the position of substituents on an aromatic ring
this may leave them better placed for interaction
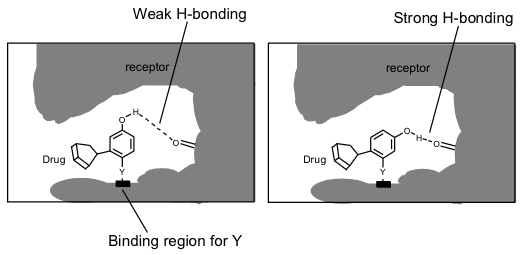
why changing position of a substituent on an aromatic ring can change efficacy of a drug
changing the position of one substituent may effect the electronic properties of another if they become ortho or para substituted to one another
what is structure extension for
a lead structure may not interact with all possible binding groups in a target. The addition of extra functionality may be used to probe for extra binding sites
the addition of extra alkyl or aryl groups may be used to find extra hydrophobic regions
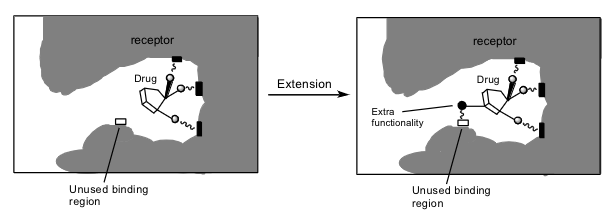
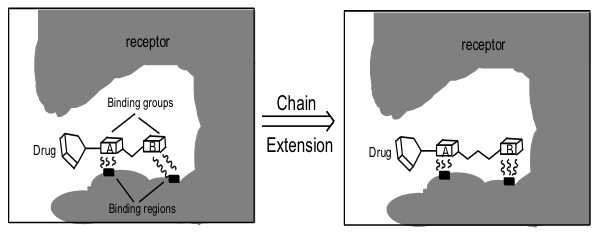
what are chain extensions / contractions
If a lead structure has two binding groups linked by a chain, the length may not be ideal for optimum binding to both sites. Varying chain length may improve drug-target interactions
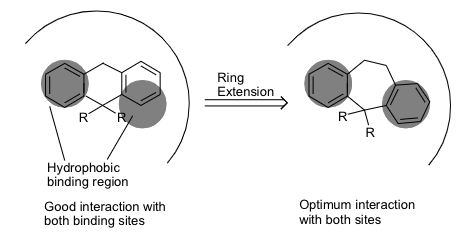
what are ring expansions / contraction
altering the size of a ring will affect the orientation of substituents - the same effect as varying substituent pattern.
Binding groups may end up in a better position to bind to the binding site
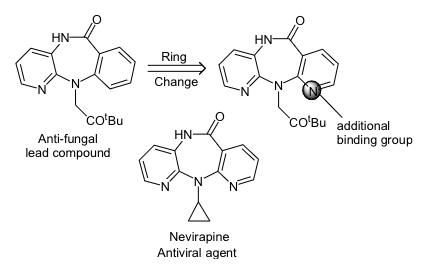
what do we mean by ring variations
a common strategy employed during analogue synthesis is to replace an aromatic ring with a different heteroaromatic ring
this may result in extra hydrogen bonding with the binding site
what do we mean by extension by ring fusion
a lead structure can also be extended by fusing one ring onto another
this may introduce selectivity (as in the case of alkyl chain extensions) and also allow for extra van der Waals interactions
What does it mean when we say structural simplicifcation
Identification of the pharmacophore can allow us to simplify the structure of analogues so that they contain only the essential binding reasons
this will make synthesis of anaolgues far easier
negative effects of simplification
change in activity - drastic changes may result in analogues that bind differently leading to different effects
loss of selectivity - simplification may also rsult in nalogues that bind differently leading to different effects
what do we mean by structural rigidifcation
flexible molecules can take on a number of conformations which may interact with a number of different recpetors
locking a molecule into only one conformation by rigidifaction may improve selectivity
a common strategy is to discover the active inding conformation and then rigidify by incorporating the skeleton into a ring
the disadvantage of this strategy is that drug targets may difficult to synthesise
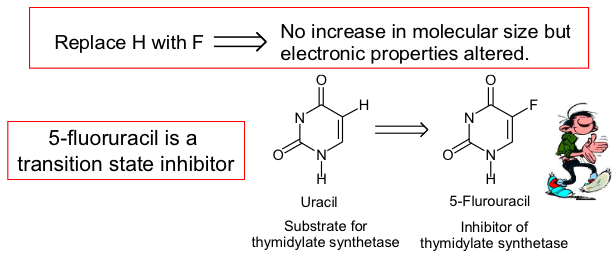
what are isosteres
an approach to analogue design is the replacement of some atoms or groups with isosteres. This can be a convenient way of altering one property of a molecule at a time, in a rational way
for example fluorine is an isostere of hydrogen. They are both the same size but fluorine is much more electronegative