unit 1: matter, chemical trends, and chemical bonding
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
39 Terms
Democritus
-first proposed indivisible particles called atoms
Dalton’s Model
Called the “billiard ball” model
Atom was a solid sphere
Atoms of same element are identical
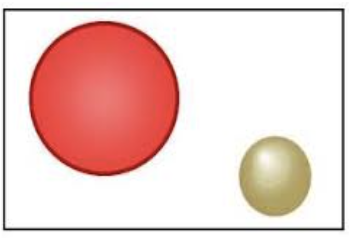
Thomson’s Model
Called the “plum pudding model”
Discovered electrons
Atom was a positively charged sphere, with negatively charged particles
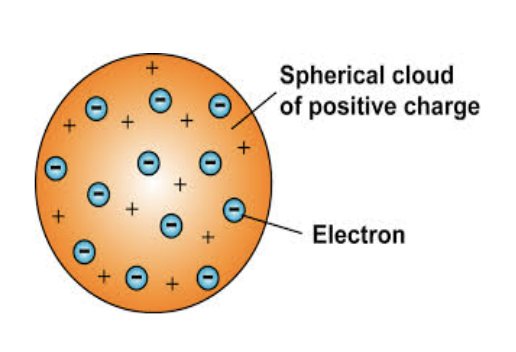
rutherfords model
Discovered nucleus
Used Gold foil experiment
piece of gold foil was hit with positive alpha particles
Most alpha particles went straight through
Showed that gold atoms were mostly empty space
Planet-like electrons orbit a positively charged nucleus
chadwick
discovered the neutron
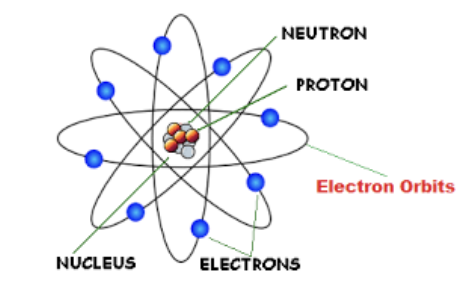
Bohr’s Model
Electrons only have a specific amount of energy,
Organized in energy levels called shells
Electrons gain/lose energy to move between shells
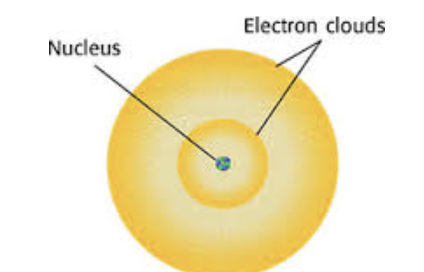
Schrodinger
Showed that electrons move in a region of space, often represented as a cloud
explaining flame tests
When an atom is subjected to heat or electricity, the electrons in the atom become excited
The electrons absorb energy and jump from the ground state to the excited state. This is called a quantum jump.
What goes up must come down. Eventually the electron in the excited state will fall back to the ground state and release energy in the form of coloured light.
quantum jump
The electrons absorb energy and jump from the ground state to the excited state. This is called a quantum jump.
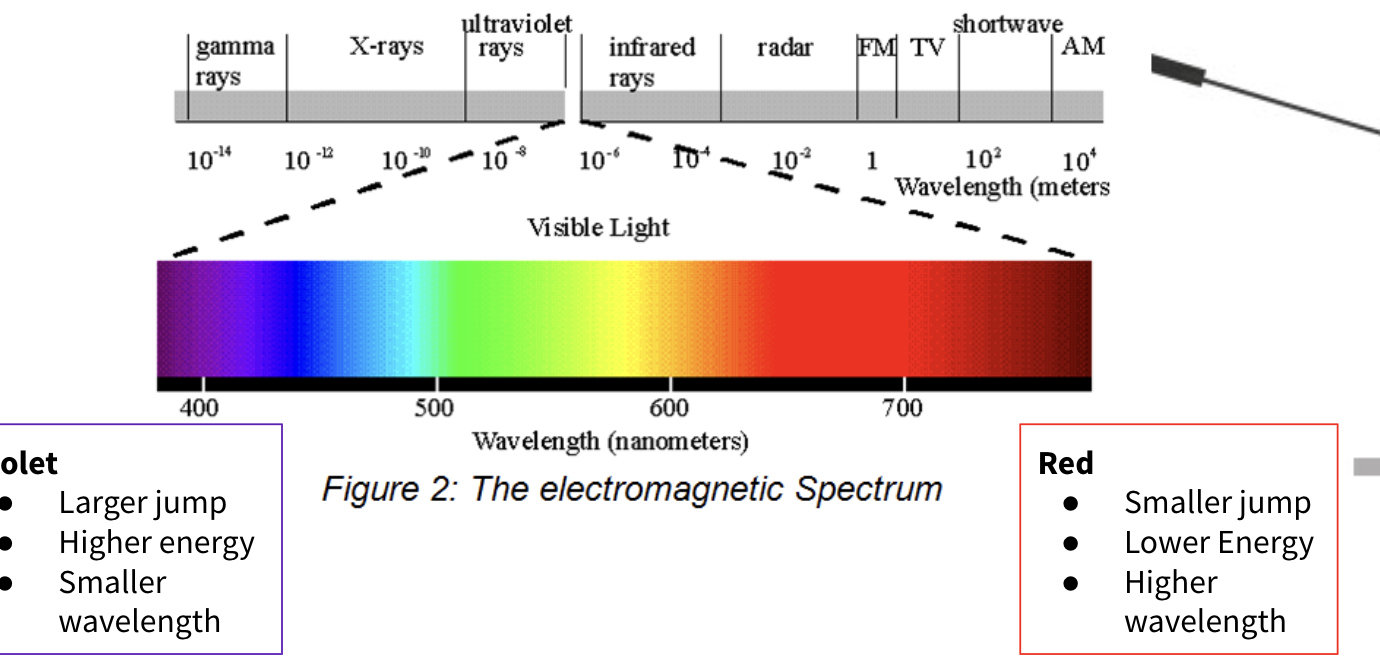
how different colours of light are created
The colour of light emitted depends on how big the jump is and thus the amount of energy released.
remember for explanation:
large/small jump
high/low energy
smaller/larger wavelength
line spectrum
a series of coloured lines separated by bands of blackness
when the coloured light is viewed through a spectroscope
unique to every element
like a fingerprint that aids in identifying the element
isoelectronic
Noble gases have 8 valence electrons and therefore a very stable shell
Helium is the exception as it is stable with only 2 valence electrons (first shell is full with 2)
Some atoms acquire stable octets by gaining or losing electrons.
Once they acquire a stable octet (usually 8 in an outer shell), they are said to be isoelectronic with the noble gas that has the same total number of electrons.
protons
positively charged particles in the nucleus
Determine the identity of the atom
electrons
Negatively charged particles
Orbit the nucleus
Transferred in chemical reactions
isotopes
Atoms of the same element that have different masses due to a different number of neutrons
same number of protons and electrons means similar chemical/physical properties
radioisotope
An unstable isotope of an element
Undergoes radioactive decay into more stable nuclei
why is atomic mass a weighted average
The existence of isotopes can explain why the atomic mass on the periodic table is an average atomic mass.
This mass is a weighted average.
chemical bonds
The forces that attract atoms to each other in compounds
electronegativity definition
A measure of an element’s ability to attract electrons in a chemical bond
electronegativity
If the electronegativity of one of the two atoms in the bond is greater than the electronegativity of the other atom, the electrons will be more strongly attracted to the first atom
Electrons spend more time around atoms with higher electronegativity
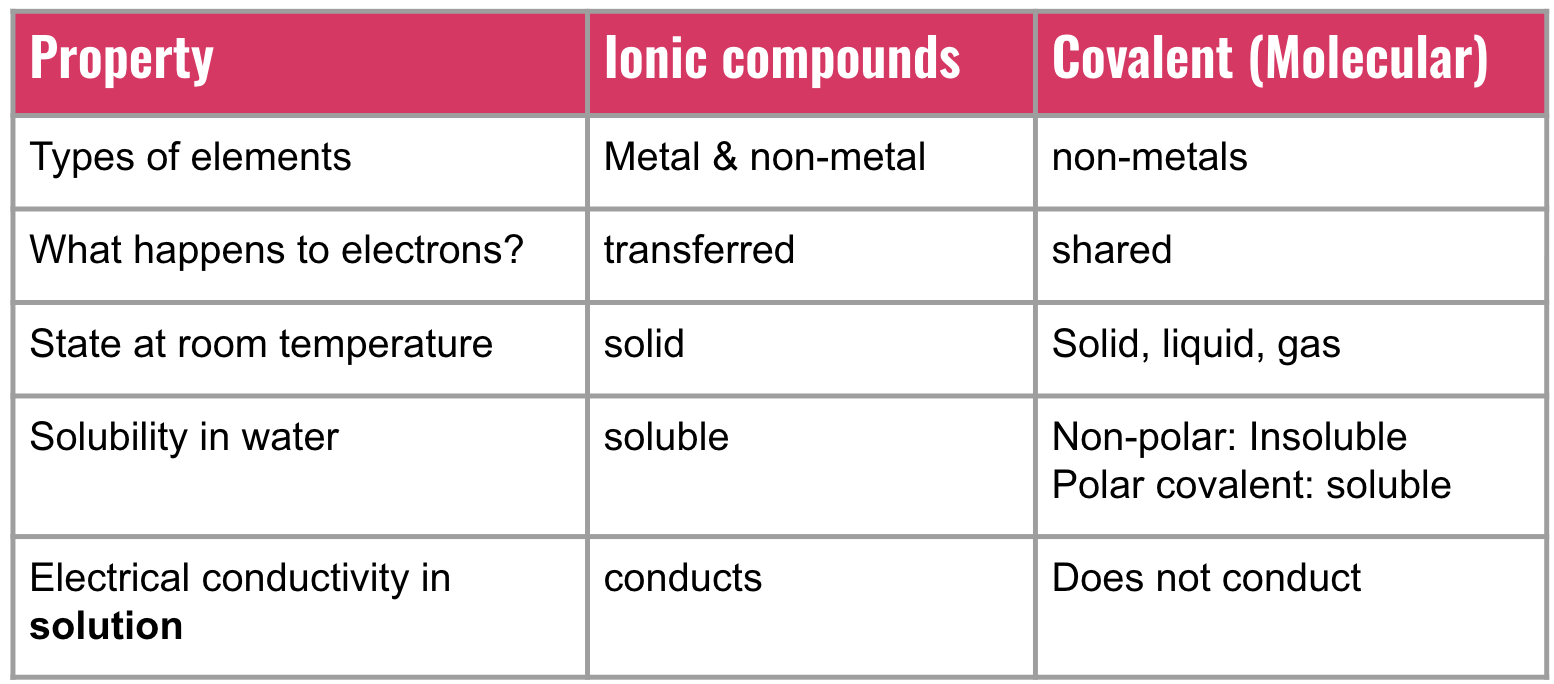
how to determine type of bond
Compare electronegativity values of two elements in a chemical bond
This determines whether they will equally share electrons (COVALENT), share electrons unequally (POLAR COVALENT), or share so unequally that they transfer electrons (IONIC).
most polar to least polar
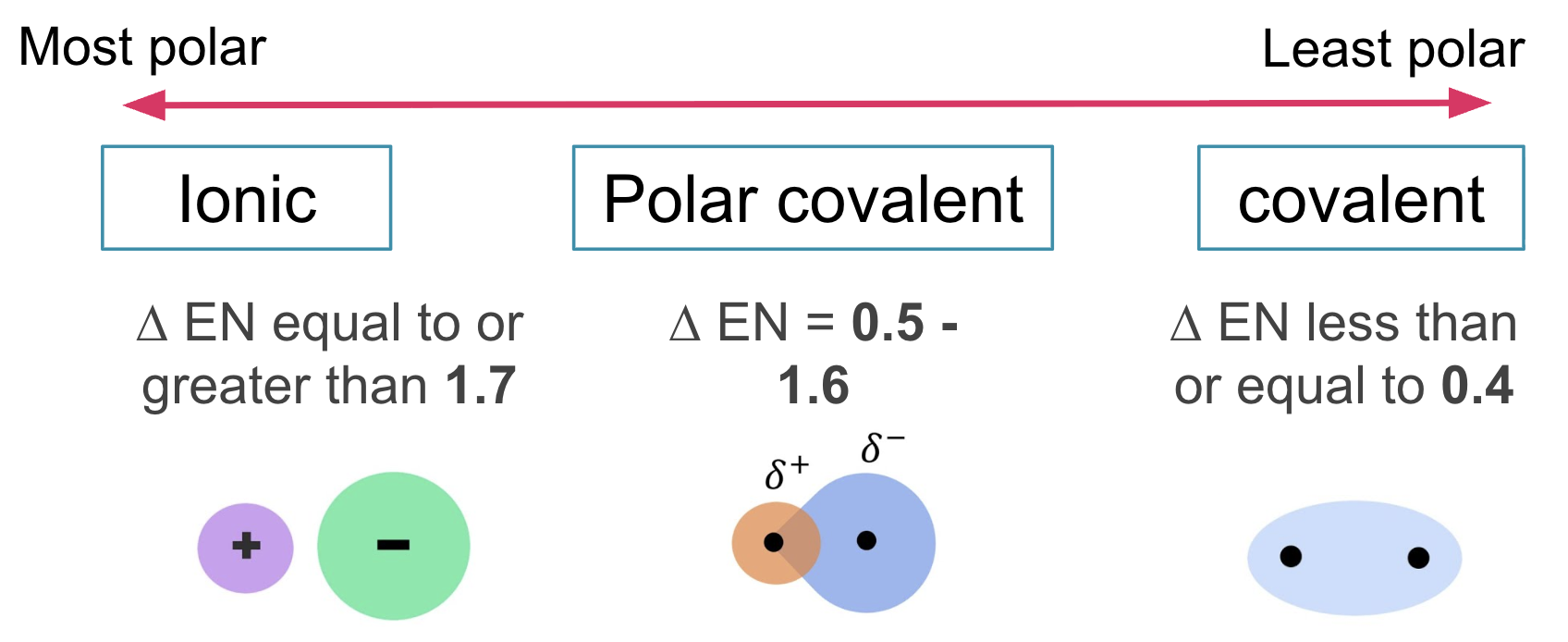
polar
uneven distribution of charge
has a partially positive (∂+) and partially negative (∂-) end (dipole)
* the atom with higher delta EN is partially negative, lower delta EN is partially positive
keeping molecules together
Opposite ends of partially charged molecules attract creating intermolecular forces
The higher the ∆EN, the higher the melting/boiling points
formation of ionic compounds
Ionic compounds transfer electrons to produce positive ions and negative ions
The opposite charges attract
Charged ions conduct electricity when dissolved in water
The attraction is strong and therefore ionic compounds:
have HIGH melting point
are solids at room temperature
lewis structure for molecular compounds
Determine, from the chemical formula, the number of atoms of each type of element in the compound.
Use the periodic table to determine the number of valence electrons for each atom. Add these up for the total number of valence electrons in the compound.
The element that requires the most bonds is the central atom. Arrange the other elements around the outside.
Place two dots between elements (bonding pairs)
Place remaining valence electrons around the atoms in pairs (lone pairs) to complete their octets, do the central atom last
If there are not enough electrons for all atoms to have 8, create double or triple bonds by sharing additional electron pairs
the modern periodic table
Modified Mendeleev’s table
Organizes elements according to atomic number
periodic law
Chemical and physical properties of elements repeat in a regular pattern when arranged by increasing atomic number
periods, families (groups), valence electrons
Periods: horizontal rows
Groups (aka families): vertical columns, same number of valence electrons gives similar properties
Valence electrons: electrons in the outermost energy level (orbit), involved in chemical reactions
when examining periodic trends…
When examining periodic trends, always look at:
# energy levels (orbits)
# protons
As we go down a group: # energy levels increase
As we go across a period (left to right): # protons increases
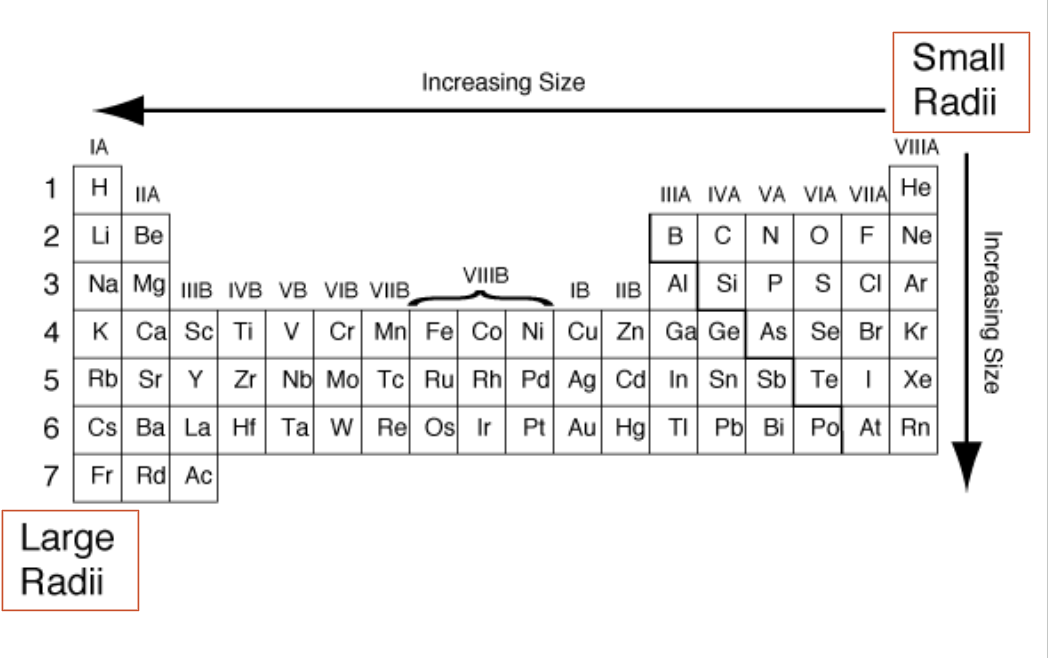
atomic radius
the size of the atom
Ex explaination: Which would be larger - Be or Mg?
Be has 2 energy levels
Mg has 3 energy levels
Mg is larger
OR
Eg. Which would have the smallest radius: Mg or Si?
Both have 3 energy levels
Si has more protons to attract the electrons closer
Si is smaller
atomic radius in general
Down a group:
AR increases (more energy levels)
Across a period:
AR decreases (more protons, same energy levels)
ionization equations
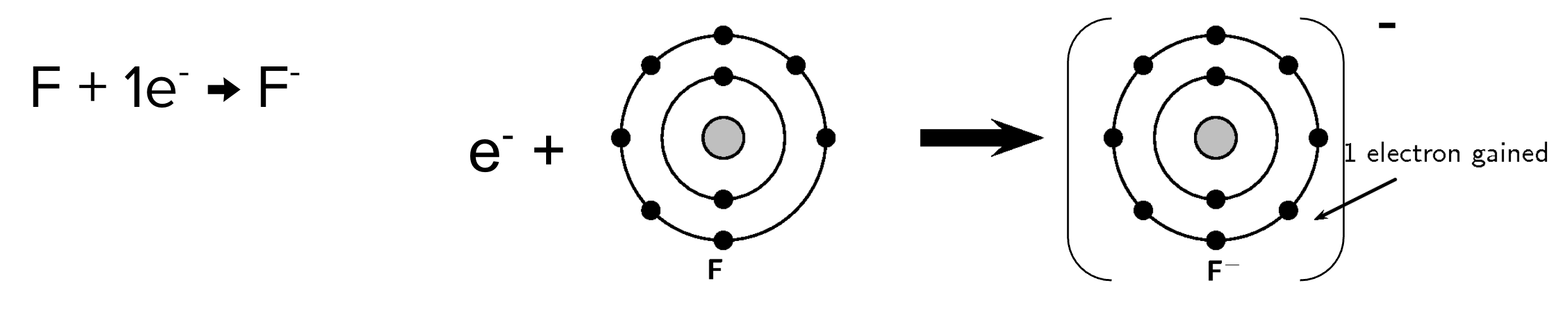
Atoms will lose or gain electrons (react) so that they are isoelectronic with the nearest noble gas
This makes the atom stable as its orbits are full
Ionization Energy
The amount of energy required to remove an electron from the outermost energy level of an atom or ion (in gaseous state)
More loosely held electrons are more Easily Removed, Lower I.E.
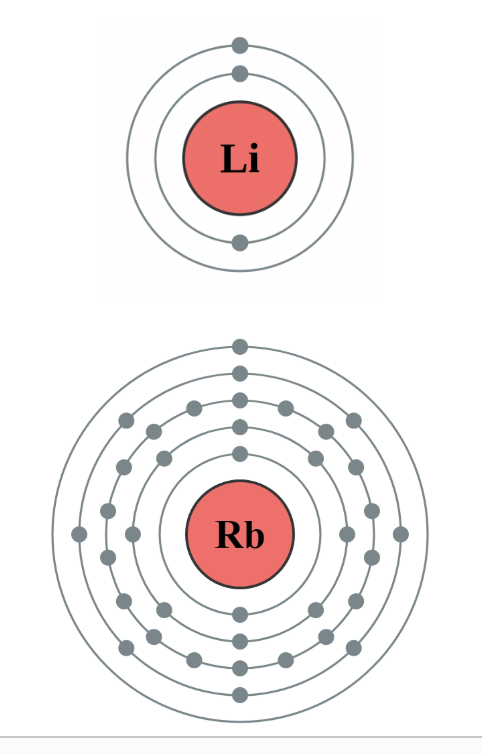
eg: Eg. Which atom has the smallest ionization energy: Li or Rb
Less energy to remove outermost electron from Rb
Negative e- farther from positive nucleus in energy level 5
Li attracts electrons more tightly because they are closer to positive pull of the nucleus in shell 2
eg: Na or Al?
Na
Same number of energy levels
Al has more protons to attract the electrons, electrons more difficult to remove, requires more energy
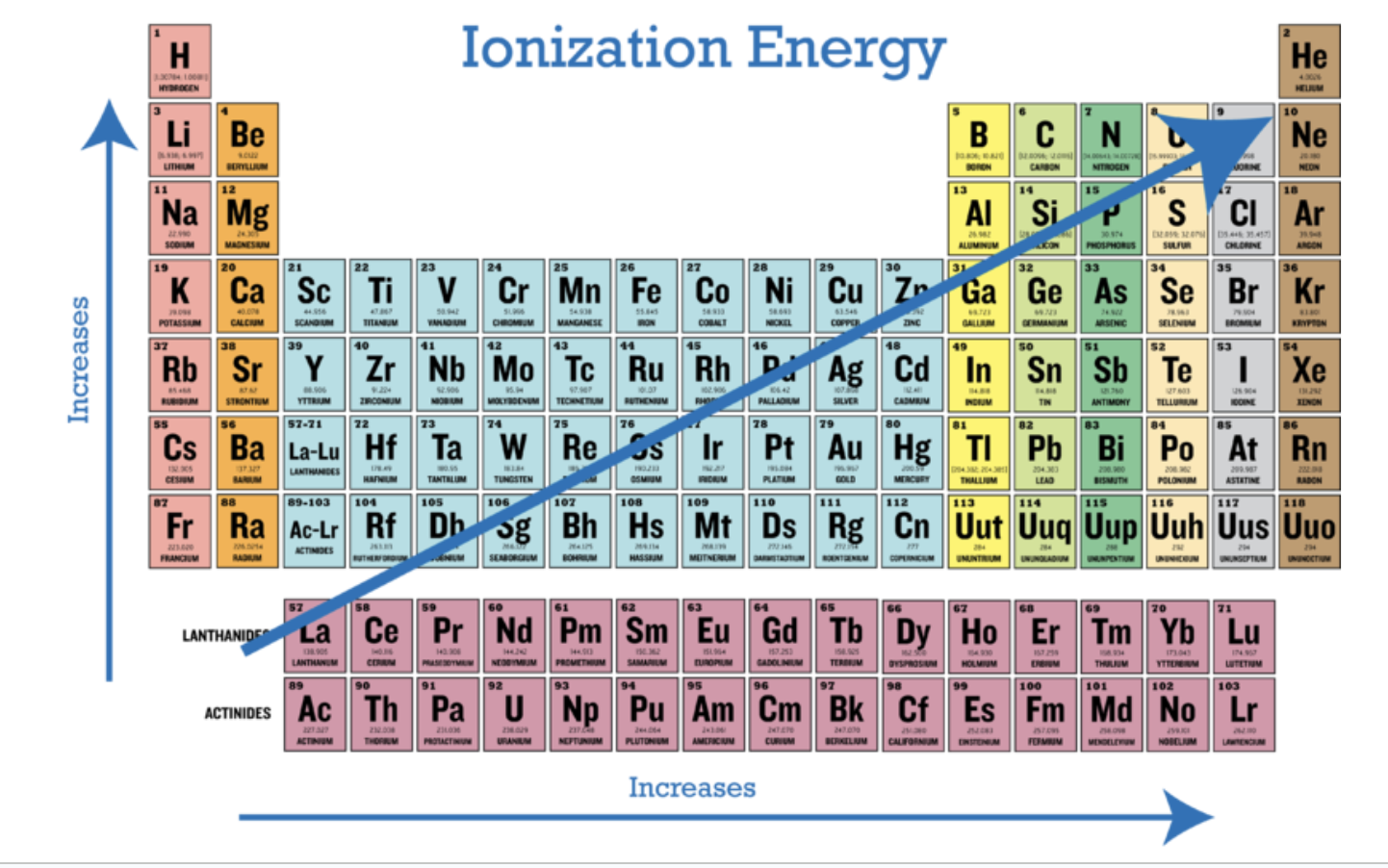
ionization energy in general
Down a group:
IE decreases (more energy levels)
Across a period:
IE increases (more protons, same energy levels)
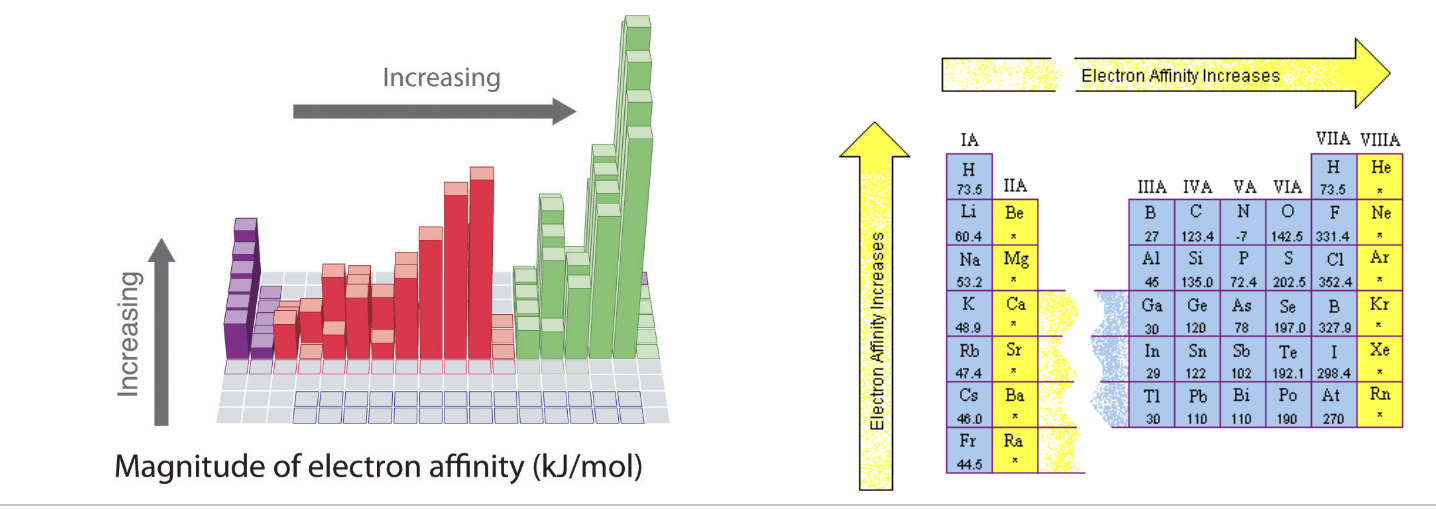
electron affinity
How much more stable an atom is after gaining an electron
Large negative numbers considered high EA
Negative sign means energy is released (hurray!)
ex: does F or O jave larger electron affinity?
Same number of energy levels
F has more protons to attract same number of energy levels
F will hold electrons more tightly, better attract new electrons
F will have larger electron affinity
electron affinity
Down a group: decreases (more energy levels)
Across a period: increases (more protons)
electronegativity
Ability of an atom to attract electrons in a bond
electronegativity in general
Down a group: EA and EN decrease (more energy levels)
Across a period: EA and EN increase (more protons)