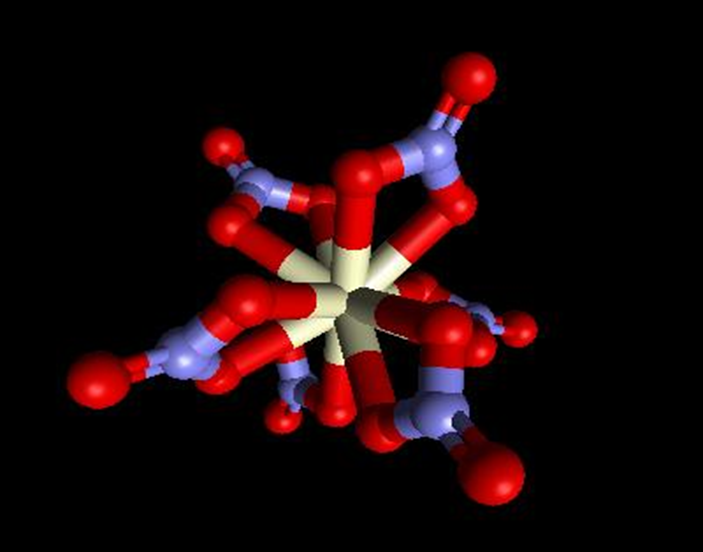
4 & 5. Coordination numbers
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
What is the inner sphere?
Inner sphere complex is the species formed only by the ligands attached directly to the central metal ion (what is between the [ ] in a complex)
![<p>Inner sphere complex is the species formed only by the ligands attached directly to the central metal ion (what is between the [ ] in a complex) </p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/85bc0015-b943-4d7d-bd29-4ef0e5af5b30.png)
What is the outer sphere?
The complex including species not directly bonded to the central metal ion - complex including things out the square bracket
E.g. When there is solvent of crystallisation ([ML6].H2O)
Or an ion pair electrostatically interacting with the complex - [M(II)L6]Cl2
These are associated species that work to balance out the ionic charges leftover if all of the ligands are neutral
What is the coordination number? What is is determined by?
The number of donor atoms
Does not have to be equal to the number of ligands as a ligand could be multidentate
CN is usually determined by the size of the central metal ion, steric interaction between ligands and the electronic interaction between central ion and ligands.
What are features of CN 1? What ions are usually part of it?
Rarely observed
Usually only ion pairs in the gas phase (Na+Cl-) or coordination entities with d10 orbitals with a highly sterically demanding ligand
E.g. [M-C6H2(Ph)3] where M = Cu(I) or Ag(I)
![<ul><li><p>Rarely observed </p></li><li><p>Usually only ion pairs in the gas phase (Na<sup>+</sup>Cl<sup>-</sup>) or coordination entities with d<sup>10</sup> orbitals with a highly sterically demanding ligand </p></li><li><p>E.g. [M-C<sub>6</sub>H<sub>2</sub>(Ph)<sub>3</sub>] where M = Cu(I) or Ag(I)</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/adb1ca85-f51d-420a-83af-3bd58db6aa5d.png)
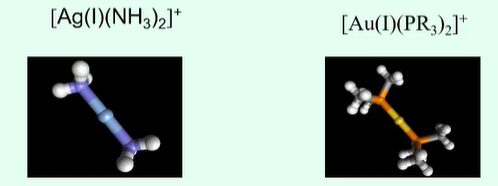
What are features of CN 2? What ions are usually part of it?
Favoured ligand arrangement will be linear (D∞h)
Usually limited to +1 ions of group 11 - d10 orbitals like Cu(I), Ag(I), Hg(II) with ligands like NH3, CN-, Cl-
CN 2 has a low stability so adding excess ligand will just result in more ligands joining the complex and it will no longer be in the 2 coordination state.
Metal cyanides are most common occurrence for CN 2
How does their linearity lower stability for CN 2 complexes?
Linearity lowers their stability through there being two large areas of attack unless the sterics can work in favour to oppose the attack
Additional ligand added to CN 2 complexes can therefore attack and change the coordination
In the image, the Au complex is more stable as the R groups are causing steric hinderance and will limit the area of attack more.
Whereas, the Ag complex only has NH3 groups will little steric hinderance leaving it open to attack and low stability.
What are features of CN 3? What ions are usually part of it?
Also quite rare with few examples - mostly found with Cu(I) (d10)
Real complex examples: [HgI3]- and [Cu{S(PMe3)}3]+
Preferred geometry of planar M-centred triangle (D3h complexes)
What are the geoemetries for Cn 4?
Square planar or Tetrahedral
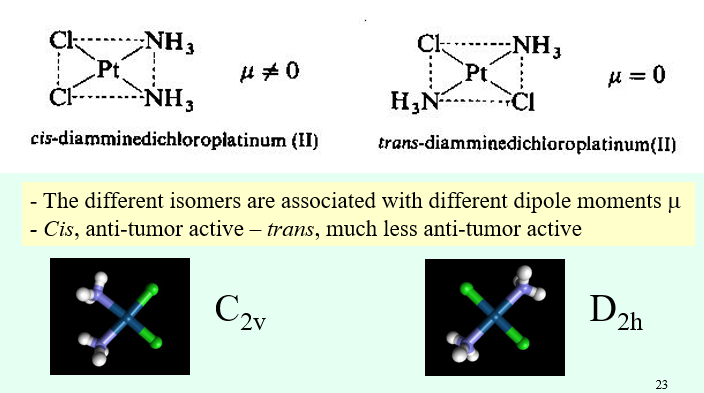
When does a CN 4 square planar complex exist? What isomerism can they exhibit?
Favoured by d8 metal ions due to crystal field theory
E.g. Ni(II), Pt(II), Au(III), Pd(II), Rh(I), Ir(I)
Can be forced by planar tetradentate ligands such as porphyrins
For steric reasons, it is only observed for smaller ligands, however is susceptible to coordination of two additional axial ligands to become an octahedral complex
Can exhibit cis-trans isomerism if there are two different ligand groups.
When does a CN 4 tetrahedral complex exist? What form of isomerism can it have?
Formed when steric crowding is important - small central atom with larger ligands like Cl-, Br- etc.
Or formed with high oxidation state metal ions (left of d-block) with oxoanions e.g. [VO4]3-, [CrO4]2-, [MnO4]-
Tetrahedral is favoured by cations with a high oxidation state e.g. Mn7+
There is possibility for chirality (optical isomerism) with tetrahedral complexes if there are four different groups - this is rarely observed however as tetrahedral complexes have high liability and will lead to fast racemisation
What geometries can Ni2+ form depending on its ligands?
Square planar with CN- as the bonding of cyano ions is a pi acceptor ligands in the plane
Tetrahedral with bulky ligands like Cl-, Br- and I-
Octahedral with less bulky ligands like H2O and NH3
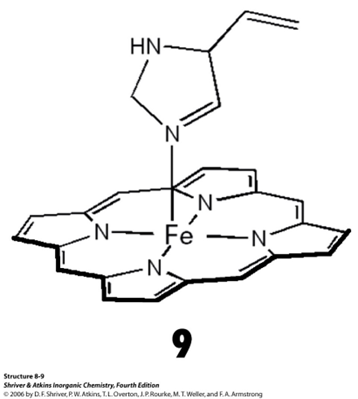
What are features of CN 5? What geometries and examples fall under CN 5?
There are only a few examples as there is high tendency of ML5 complexes to dissociate: 2ML5 = ML4x+ + ML6x-
Will either forma a square pyramidal (C4v) or a trigonal bipyramidal (D3h) complex
The active centre of myoglobin for the oxygen transport to protein is a real world example of CN 5 complex
Some polydentate ligands may induce a trigonal bipyramidal shape due to their own sterics - these are called tripodal ligands
How can square pyramidal (SP) and trigonal bipyramidal (TBP) shift and bend?
Described as distorted TBP or SP and the tau index is used to measure the index, the closer to 0 or to 1 decides if the complex will be closer to distorted TBP or SP.
TBP will be favoured based on pure electrostatic considerations, however ligands may dictate a particular geometry.
How do the energies of SP and TBP complexes differ?
in many cases they are very similar in energy
Some solids state cases show SP and TBP forms will occur in the same crystal as they are so close in energy
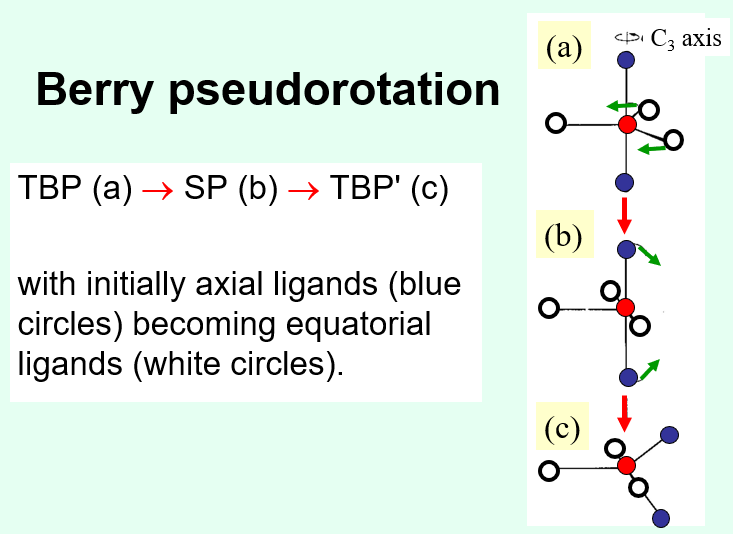
In solution, TBP complexes with monodentate ligands will be highly fluxional (able to twist into different shapes), which is the berry pseudo-rotation mechanism.
Axial ligands in a TBP complex rotate and become equatorial forming an SP complex and they rotate once more to become TBP again in a slightly different atom arrangement.
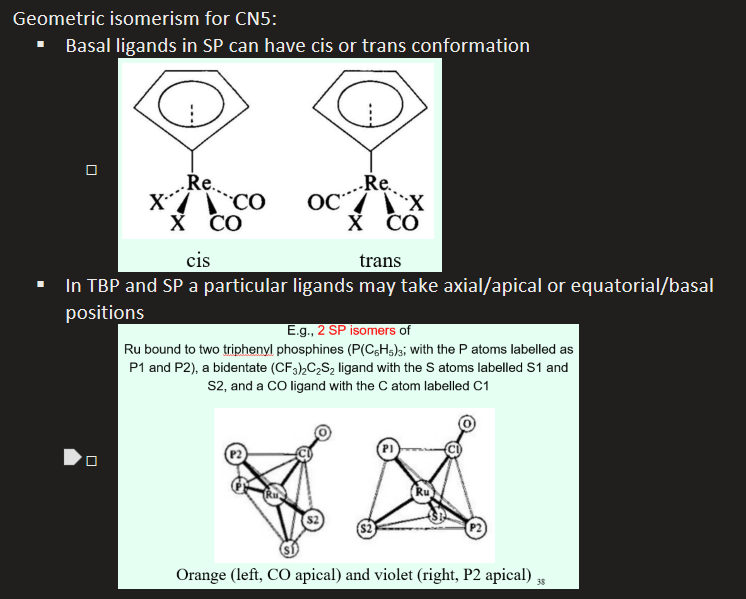
What geometric isomerism can occur in CN5 complexes?
Basal ligands in SP complexes can have cis or trans conformation
There will also be geometrical isomers depending on if particular ligands take axial/apical or the basasl/equatorial positions
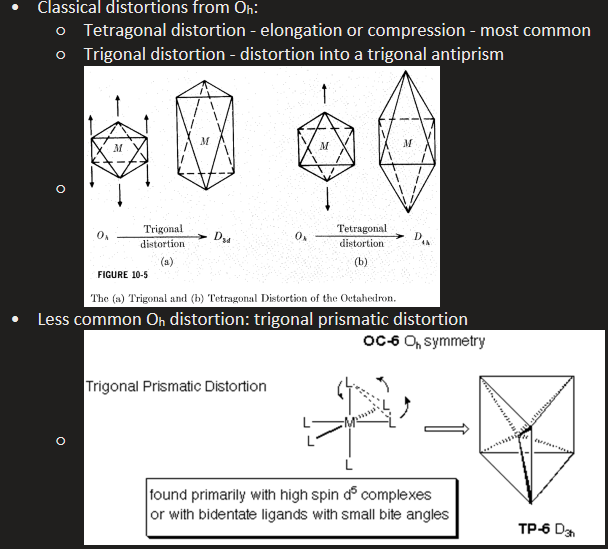
What are features of CN 6? What geometries fall under CN 6?
Most frequently occurring coordination number, most often seen with 2+ or 3+ metal ions
Most often octahedral geometry - however there are three distortions
Tetragonal distortion - elongation or compression of ligands (from Oh to D3d)
Tetragonal distortion - axial ligands stretch and complex is distorted to trigonal antiprism (Oh to D4h)
Less common distortion - Trigonal prismatic distortion - Oh to D3h symmetry - primarily found with high spin d5 complexes or with bidentate ligands with small bite angles
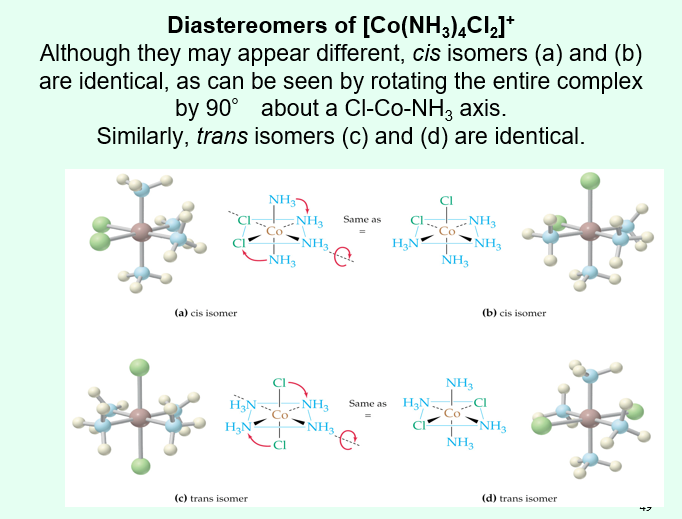
Do CN 6/octahedral complexes exhibit cis/trans isomerism?
Yes if there are 2 of one ligand and 4 of another ligand group (MA2B4)
There are not diastereomers however as the what looks like a different cis/trans isomer will actually be identical to the other cis/trans isomer shown through rotation
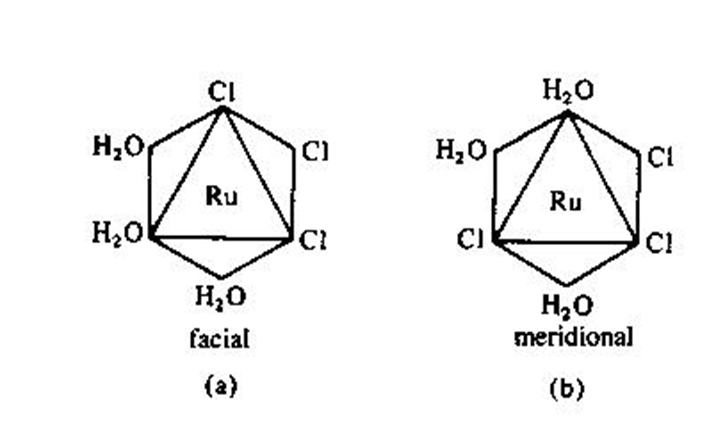
What CN 6/octahedral complex will form facial or meridional isomers, explain this form of isomerism?
MA3B3 form of CN 6 complexes will form facial or meridional isomers
Facial is when the same three ligands occupy the corners of a triangle forming a ‘face’
meridional is when the ligands form a line/row in the octahedral (axial to equatorial to axial, H2O forms this line in the meridional isomer)
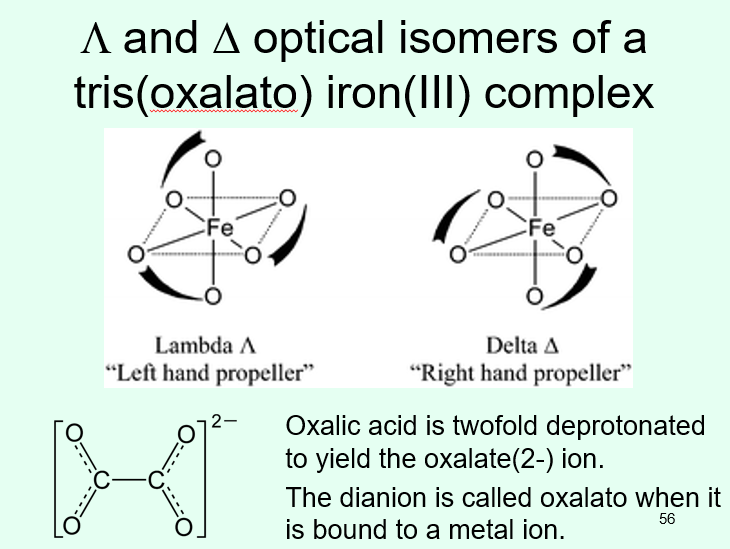
How are CN 6 optical isomers exhibited/forced?
ligands can be forced to flip to cause optical isomerism with a mirror plane
Λ and Δ can be used to label some optical isomers based on their rotational direction when there are bidentate ligands
Δ is the ‘right hand propellor’ and Λ the ‘left hand propellor’
in the example, there are three oxalato anions, each with two oxygens bound to the metal ion
What is the most common way of finding CN 7, 8 and 9?
Any coordination number larger than 6 will be for a lanthanide ion (f-block) - CN doesn’t increases endlessly due to sterics, which is why only lanthanides have the room for more than 6 ligands.
An example for CN 7 and 8 is [YB(acac)3(H2O)] and [YB(acac)3(H2O)2] respectively - acac is bidentate
For CN 9, it is often just water molecules for example: [Nd(H2O)9]3+
What is an example of a CN 12 complex?
[Ce(NO3)6]2-