Chapter 13: Parasitism
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
Parasitism and Host Relationship
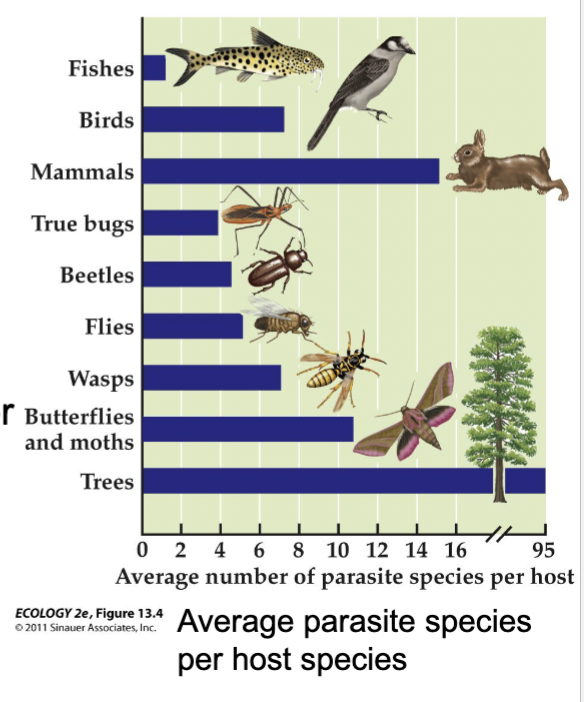
Parasites typically feed on only one or a few host species, but host species have multiple parasite species.
How effective is parasitism as a life strategy
Parasitism is an extremely effective life strategy, having evolved as many as 223 time in the animal kingdom
How many hosts do parasites usually attack?
Usually one or a few host species in their life cycle
Explain why many hosts species are host to more than one specialized parasite species
It is unusual for any host to contain the full number of potential parasite species at once
Graph included only helminth parasites (roundworm, tapeworm, flukes)
In the graph, the unusual number of parasites from trees includes many species of insects that feed on the leaves and sap of trees (some consider these insect parasites)
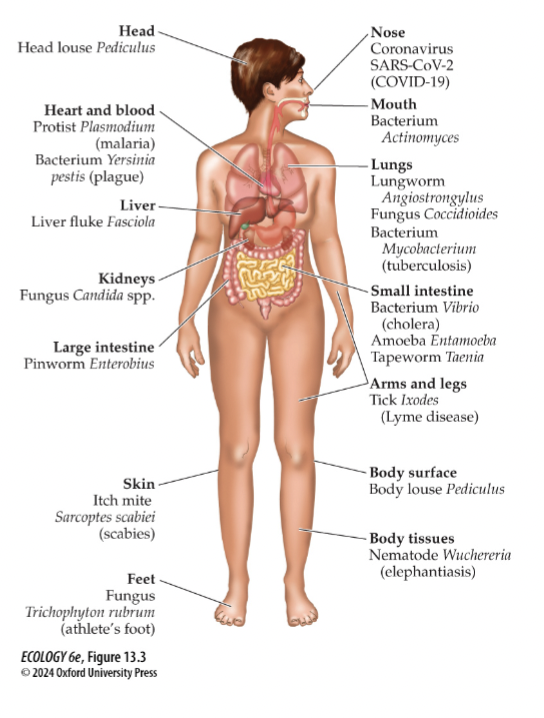
Explain how parasites specialize on many different parts of the body both internally and externally
This is possible because parasites tend to be much smaller than their hosts
On possibility is that extreme specialization evolved to minimize competition among different species of parasites for resources provided by the host (a kind of resource partitioning).
Macroparasites
Large species such as arthropods and tape worms
Img: Whale tapeworm Tetragonoporusy calyptocephalus is a giant
parasite that lives in the intestines of whales and can reach 130 feet long

Microparasites
Microscopic such as bacteria and viruses
Virus is an Obligate intracellular parasite
obligate intracellular parasite
Cannot replicate or reproduce outside of a host cell, relying instead on the host’s cellular machinery
Ex: Virus
Ectoparasites
Live on the outer body of the host

Endoparasites
Live inside their hosts, within cells or tissues, or in the alimentary canal
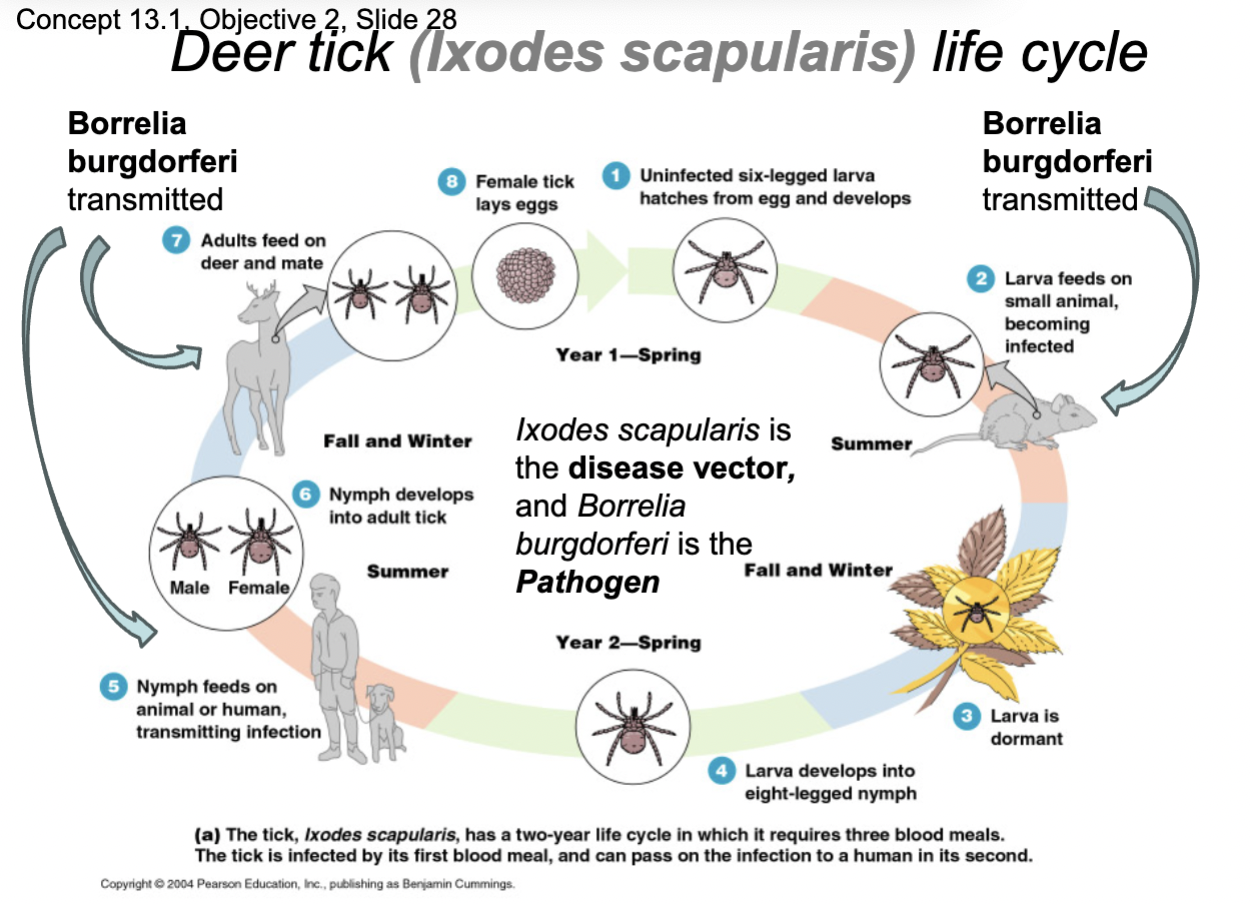
Ex: Primary parasite vector and pathogen associated with Lyme disease Borrelia Burgdorferi bacteria
Lyme Disease Vector for Humans
Blacklegged deer tick (Ixodes scapularis)
Lyme disease causative agent
Borrelia burgdorferi

Lyme Disease Symptoms
Chronic joint inflammation (Lyme arthritis), particularly of
the knee.Neurological symptoms, such as facial palsy and
neuropathy.Cognitive defects, such as impaired memory.
Heart rhythm irregularities.
Life Cycle of Deer Tick (Ixodes Scapularis)
Y1 Spring- Uninfected six-legged larva hatches from egg and develops
Y1 Summer- Larva feeds on small animal, becoming infected, Borrelia burgdorferi is transmitted
Y1 Fall and Winter- Larva is dormant
Y2 Spring- Larva develops into eight-legged nymph
Y2 Summer Nymph feeds on animal or human, transmitting Borrelia burgdorfei
Y2 Fall and Winter- Nymph develops into adult tick
Y2 Fall and Winter- Adults feed on deer and mate, transmitting Borrelia burgdorfei
Y2 Spring- Female tick lays eggs
Overall: 2 yr life cycle and 3 blood meals, tick is infected by its first blood meal and can pass the pathogen to a human in its second
2 Advantages of Ectoparasitism
Ease of dispersal
Safe from host’s immune system
3 Disadvantages of Ectoparasitism
Vulnerability to natural enemies
Exposure to external environment
Feeding more difficult
3 advantages of Endoparasitism
Ease of feeding
Protected from external environment
Safer from natural enemies
2 disadvantages of Endoparasitism
Vulnerability to host’s immune system
Dispersal more difficult
3 significant challenges endoparasite dispersal presents
Often disperse through hostile environments between multiple hosts specific to certain life stages
Produces enormous numbers of offspring to compensate for very high mortality rates associated with their dispersal
Asexual reproduction is common due to low probability of finding sexual mates (they don’t tend to move once in body)
Primary host
Also known as definitive host
Is where the parasite reaches its adult stage and reproduces sexually
Secondary Host
Also known as intermediate host
Is where the parasite develops or undergoes a life cycle phase but does not reach sexual maturity
Example of how some parasites have evolved to alter the behavior of their hosts in ways that promote the completion of their life cycle
Horsehair worms (Nematomorpha) manipulate host grasshoppers and cricket behavior to enter the water, where they drown
The worms then emerge, breed, and lay their eggs

Example of how many parasites manipulate primary hosts to attract secondary hosts
Leucochloridium paradoxum is a parasitic flatworm.
Intermediate hosts are land snails.
The pulsating, green broodsacs fill the eye stalks of the snail, purportedly attracting predation by birds, the flatworm’s primary host
Snails eat eggs in bird feces.

CC How Toxoplasma gondii makes rodents fearless of cats
In humans causes Influenza-like symptoms, swollen lymph nodes,
headaches, fever, and fatigue, but symptoms are often not readily observable.As high as 30% of human population around world may have been exposed and may therefore be infected (called latent infection).
Interesting fact: known to remove rodents' innate fear of cats.
Toxoplasma gondii is another parasitic protozoa.
Transmitted from feces of cats (the only known host in which reproduction occurs) to rodents and other mammals (including humans).
What is the host-parasite interaction representing a battle of
Parasite virulence and host defenses
4 Host defenses
Physical defenses- e.g tough skin
Immune system responses- Specialized cells to identify, engulf, and destroy microparasites
Encapsulations- Blood forms capsules around smaller parasites (common in insects)
Biochemical Defenses- Secondary compounds, common in plants
Immune system responses as host defense against parasites
Specialized cells to identify, engulf, and destroy microparasites
Encapsulation response as host defense against parasites
Blood forms capsules around smaller parasites (more common in smaller parasites)
Biochemical defenses as host response against parasites
Secondary compounds, common in plants
2 Parasite virulence (harm their hosts by decreasing their fitness)
Parasites try to multiply rapidly before an immune response can be deployed
Under selective pressure not to be so virulent as to kill their host, because the host must stay alive at least until transmitting offspring to new host.
3 ways and examples for how parasites can circumventing host’s immune system is common parasite survival strategy inside body
Parasites suppress the host’s immune system
HIV virus (Causes AIDS)
Parasites coat themselves with proteins that mimics the host’s own proteins
Ex: Schistosoma Mansoni, a parasitic blood fluke causing Schistosomiasis, or snail fever
Parasites coat themselves with novel proteins
Ex: Trypanosoma brucei, flagellated protozoan parasites that cause fatal African Sleeping sickness
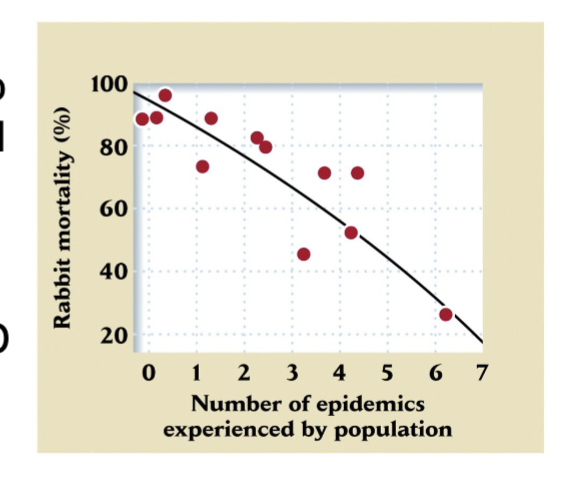
Myxoma Virus
Introduced in the 1950s and spread by mosquitoes proved to be an effective biological control agent, killing 99.8% of infected rabbits
CC How Introduction of Specialized Parasites to control invasive rabbit populations results in examples of coevolution
Rabbits introduced by British to ranch in Victoria, Australia,1859.
Years later, population exploded to hundreds of millions across continent, destroying pasturelands and threatening wool production.
Myxoma virus introduced in 1950 and spread by mosquitoes proved to be an effective biological control agent, killing 99.8% of infected rabbits.
Later outbreaks of the virus were less effective

Explain Coevolution Response Between Myxoma virus and Rabbits
Decline in lethality of the myxoma virus in Australia resulted from evolutionary response in both the rabbit and virus populations
Rabbits evolved resistance to virus (some populations had resistant genotypes)
Myxoma virus evolved to be less virulent over time (like COVID and Influenza viruses in humans)
Eventually, most surviving rabbits were resistant to virus
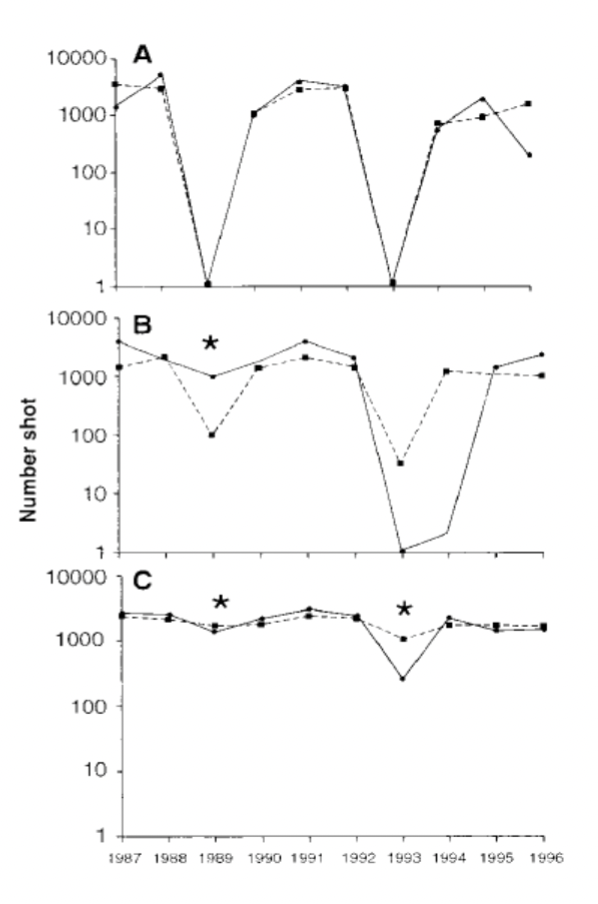
Examples of How Parasites are suspected of causing population cycles
Grouse populations exhibited population crashes approximately every 4 years. The hypothesis was that these cycles were caused by parasites.
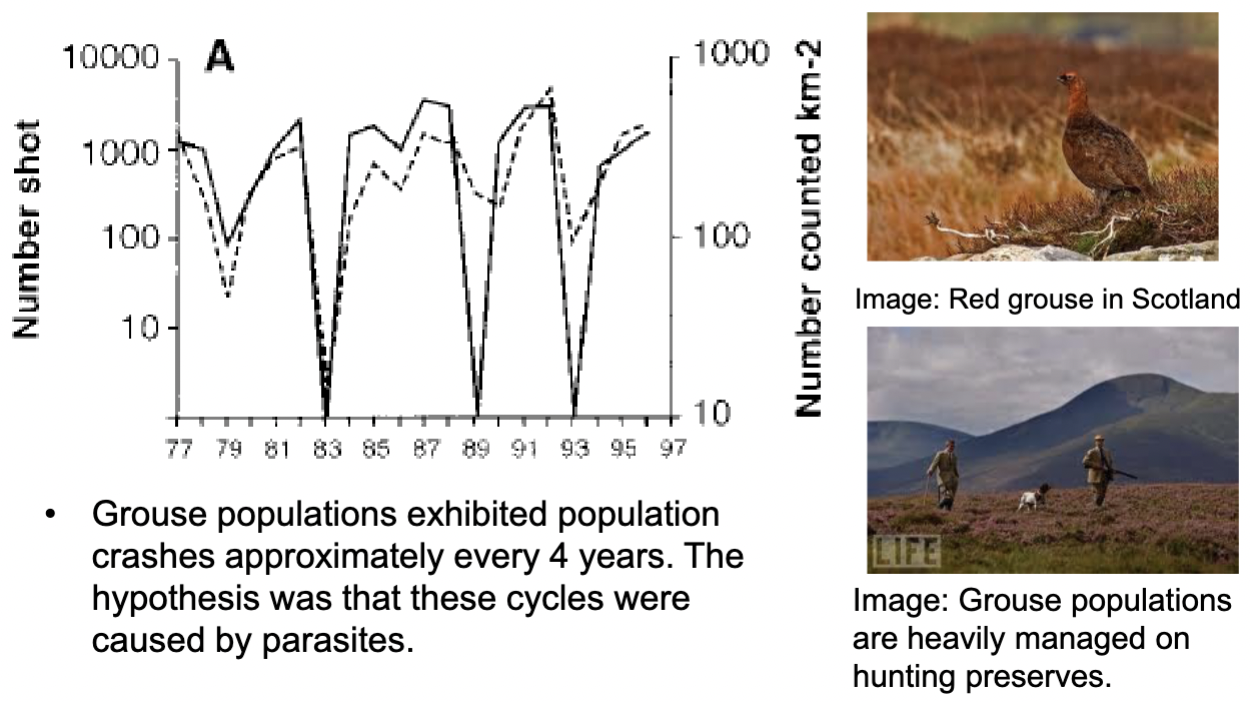
Explain the experiments that provided strong evidence that they were correct of parasites causing population cycles
Controls, no parasites removed, cycles persist.
B. Parasites treated/removed once at time indicated by*, cycles reoccur after treatment stops
C. Parasites treated/removed twice at times indicated by*, cycles eliminated.
Chytridiomycosis
is an infectious disease in amphibians caused by the chytrid fungi Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans.
Explain how Chytrid fungus pose a new threat to amphibian populations
Chytridiomycosis has been linked to amphibian population declines around the world and is spreading.
The fungus is capable of causing low to medium rates of death in some amphibian populations and 100% mortality in others.
No effective measure is known for control of the disease in wild populations.

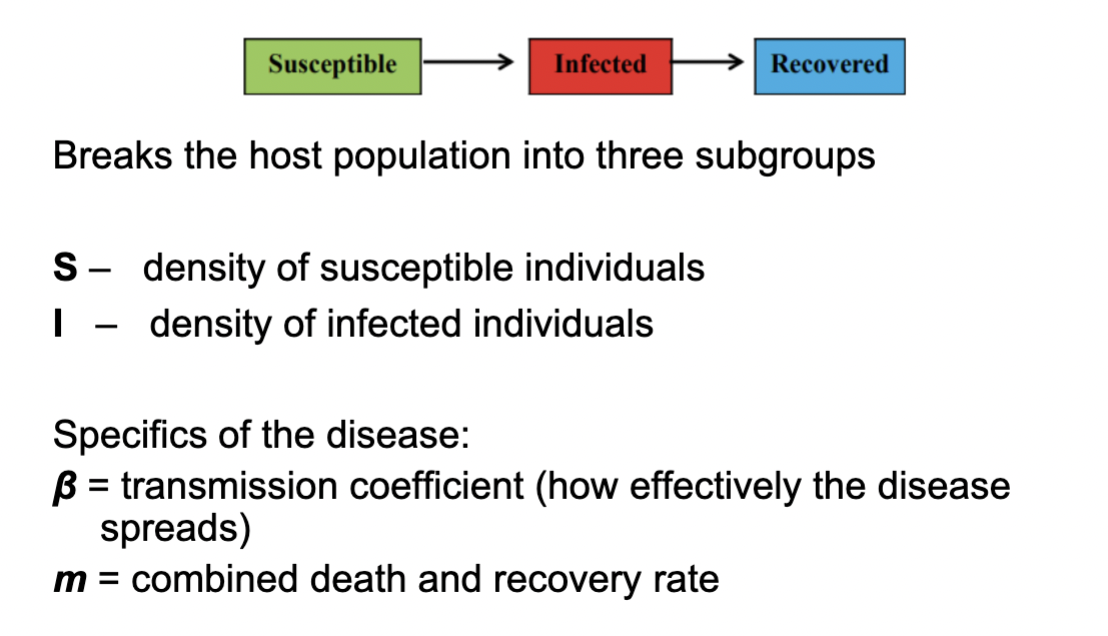
Function of Simple SIR model for Host-Pathogen dynamics
provides important insights for controlling the establishment and spread of infectious disease.
When is the only time a disease can spread?
If density of susceptible host exceeds THRESHOLD DENSITY
Components of SIR Model For host-pathogen dynamics
Breaks the host population into 3 subgroups-
S- density of susceptible individuals
I- density of infected individuals
m- recovered/death individuals
What is the SI variable meaning in SIR Model for host-pathogen dynamics
Rate of susceptible encountering infected individual
For a disease to spread, infected individuals must encounter susceptible individuals (S x I)
What is the β variable meaning in SIR Model for host-pathogen dynamics
Transmission Rate
(i.e how effectively the disease spreads from infected to susceptible individual)
βSI in SIR Model for host-pathogen dynamics
Transmission rate of disease (β) x The rate of susceptible individual encountering infected (SI)
The density of infected individuals increases when disease is successfully transmitted (at rate βSI)
And decreases when infected individuals die or recover from disease
mI in SIR Model for host-pathogen dynamics
The rate at which individuals die or recover is equal to the density of infected individuals multiplied by the death/recovery rate
Rate of decrease in density of infections
S in SIR Model for host-pathogen dynamics formula
Susceptible density
I in SIR Model for host-pathogen dynamics formula
Infected density
β in SIR Model for host-pathogen dynamics formula
Transmission coefficent
m in SIR Model for host-pathogen dynamics formula
Death and recovery rate
(dI/dt) in SIR Model for host-pathogen dynamics formula
Change in density of infected individuals at time
What does the SIR Model for host-pathogen dynamics model equation show:
Rate of change in density of infected individuals at time t is equal to the rate of increase in density of infections minus the rate of decrease in density of infections.
(dI/dt)= βSI-mI
(dI/dt)= Rate of change in density of infected individuals at time t
βSI= Rate of increase in density of infections
mI- Rate of decrease in density of infections
When is disease established and spread in a population
When density of infected individuals in a population increases over time
This occurs when (dI/dt) > 0 or βSI- mI > 0
Will become established and spread when the number of susceptible individuals (S) exceeds m/β
Therefore disease is established as spread when S(t) = m/β
Threshold Density
Formula: S(t)= m/β.
To stop the spread of the disease, we need to keep the density of susceptible individuals (S) below
3 ways to control spread of disease
To stop the spread of the disease, we need to reduce below threshold density, S(t)
1. Lower the density of susceptible individuals (S)Slaughter susceptible animals (a common practice when
the disease is transmissible to humans)Vaccines (extremely effective when available, but costly)
2. Increase the recovery rate (m) by improving early detection
and treatment.3. Increasing threshold density by decreasing transmission rate (β) by quarantining infected individuals which makes it difficult for ideas to spread