Scientific measurement (1-4 notes)
1/7
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms

Qualitative measurements
Measurements that are descriptive but not true measurements (examples include hot and large)


Quantitative measurements
Measurements that are descriptive with numbers and units


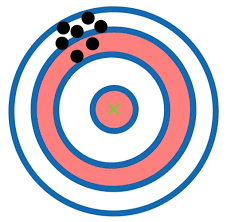
Accuracy
How close the measurement is to the actual value (this can be determined with only one measurement)

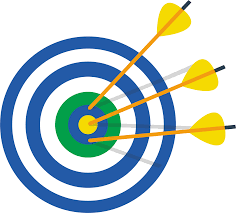
Precision
How well a certain measurement can be repeated (multiple measurements are necessary to determine this)

Scientific notation
A convenient way to express very large and very small numbers (numbers are expressed as the product of a coefficient and 10 expressed to a power)
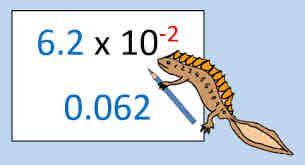

Significant figures (sig figs)
The meaningful digits in a measurement

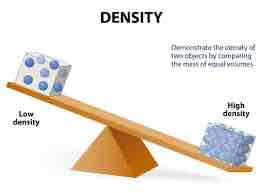
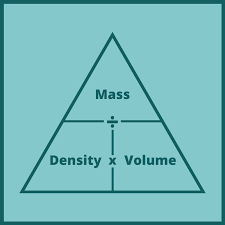
Density
The ratio of mass to volume for a sample of matter

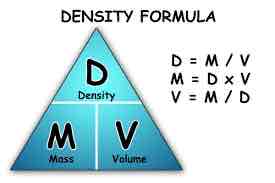
Density formula
Density = Mass / Volume