Chap 3 - Atomic structure
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
State the electric charge, relative mass and mass of proton, neutrons and electrons
Proton : within nucleus +1
Electric charge : +1.60x10^-19
Relative mass : 1
Mass: 1.67×10^-27
Neutron : within nucleus 0
Electric charge : 0
Relative mass : 1
Mass: 1.67×10^-27
Electron : revolve around nucleus at high speed -1
Electric charge : -1.60x10^-19
Relative mass: 1/1840
Mass: 9.11x10^-31
What is an atom
Atoms - smallest unit of an element (made of subatomic particles)
held together by electrostatic force of attraction between proton and electron due to opposite charges
nucleon no. = mass no. = proton+neutron
Atomic no. = proton no.
What is an isotope
Atoms of the same element with the same number of protons but different number of neutrons
Diff physical properties (mass density) , same chemical properties (same number of electrons which are involved in chemical reactions)
May be stable or unstable (radioactive)
Explain how to calculate the angle of deflection
Angle of deflection = | size of charge q / mass m |
Why is a heavier particle more difficult to deflect than a lighter one?
Assuming velocity of particles are the same, a heavier particle enters the electric field with more KE, for the same work done by electric field, it deflects less
What is the principal quantum number
Principal quantum number n = main energy level of an electron
Indicates the relative distance of electron from neutron
Smaller the n -> closer the shell is to nucleus -> stronger electrostatic attraction between any electron occupying the shell and positive nucleus -> electron more stable (lower energy)
What is a sub shell
Describes distribution and energy of electrons within each electron shell
Electrons in the same subshell have the same energy level (degenerate)
Describe orbitals
Def. : Regions of space where electrons are likely to be found (90% probability)
Each subshell contains one or more orbitals at same energy level but different orientations
Each orbital can contain up to a maximum 2 electrons
State the number of orbitals and max number of electrons in each sub shell
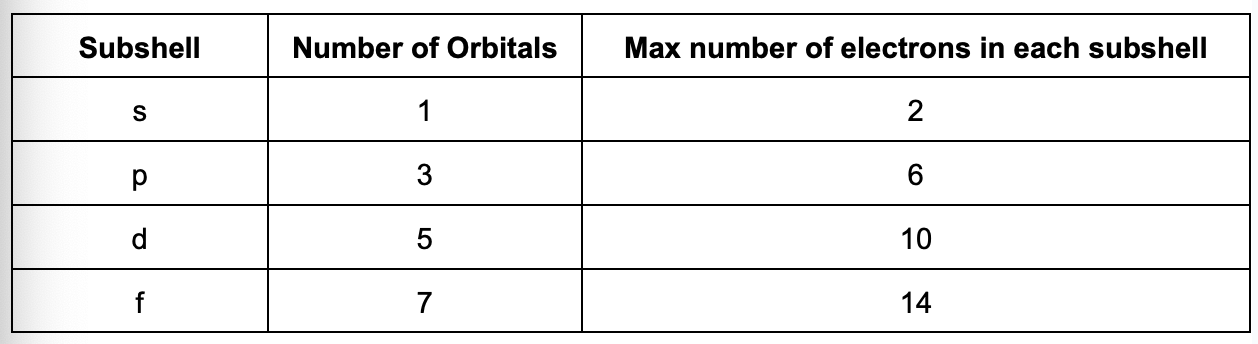
Describe the s sub shell
Spherical in shape with no nodal plane (region of 0 electron density)
S-orbitals of different shells have same shape but different size
Size of s orbitals increases with principal quantum number
Probability of finding an electron at a given distance from the nucleus is the same regardless of the direction from the nucleus
Draw the s orbital
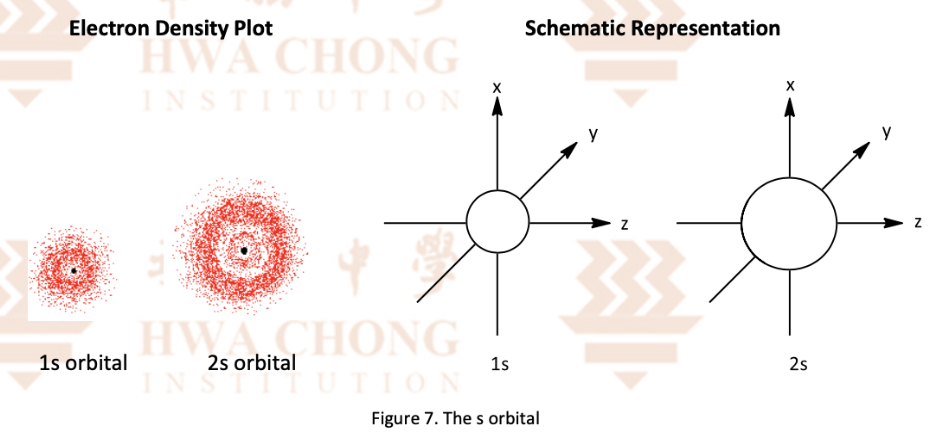
Describe the p sub shell
Dumbbell shape
P orbitals have same shape and size
P orbitals of different shells have the same shape but different size
Each p orbital consists of 2 lobes with a region of 0 electron density between them centered on the nucleus
Labelled npx , npy , npx (n = quantum number, x,y,z = axes)
Size of p orbitals increases with principal quantum number
Draw the p sub shells
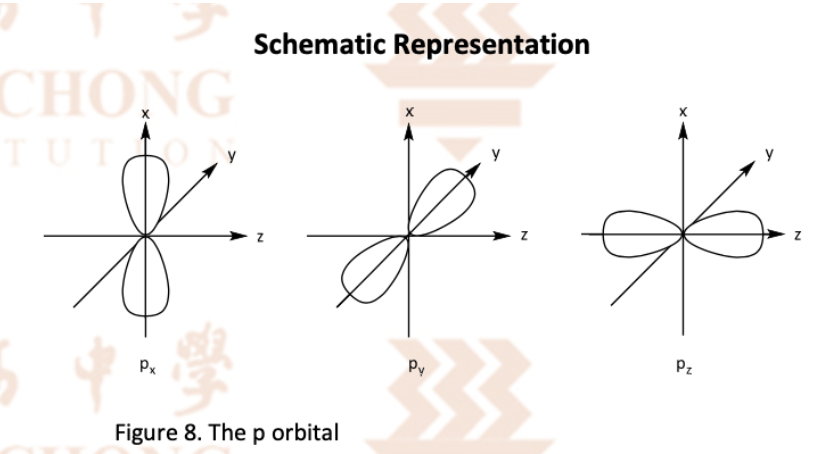
Describe the d orbitals
5 d orbitals also degenerate
4 of 5 have the same shape (made of 4 lobes, centred at nucleus) but differ in orientation in space
Remaining ndz^2 orbital is uniquely shaped among the 5 d-orbitals
Size of d orbitals increases with principal quantum number
Draw the 5 d orbitals
Describe the relative energies of orbitals
Closer orbital to nucleus, lower its energy
Energy of orbitals increase with increasing n (some overlap between energies of certain orbitals)
Increases in order of s , p , d , f
Orbitals of same subshell have equal energy (degenerate orbitals)
Describe the 1st rule when deciding electronic configuration
Rule 1 : The Aufbau / ‘Building-up’ principle
Orbital with lowest energy is always filled first before filling orbitals of higher energy
Order: 1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d → 5p → 6s → 4f → 5d → 6p → 7s → 5f → 6d → 7p
Eg. Nitrogen will have 2 electrons in 1s orbital and 2s orbital and 3 electrons in 2p orbital (total = 7 electrons)
Electronic configuration : 1s2 2s2 2p3
4s orbital filled before 3d orbital -> empty 4s has lower energy level than 3d orbitals
When removing electrons from the filled orbitals of an atom to form ions, 4s electrons removed first before 3d electrons as 3d subshell is closer to nucleus
Once 3d subshell is occupied by electrons, they repel the 4s electrons further from the nucleus causing 4s electrons to have a higher energy level
Describe the 2nd rule when deciding electronic configuration
Rule 2 : Pauli Exclusion Principle
2 electrons sharing the same orbital must have opposite spins
2 electrons exhibit sufficient magnetic attraction to hold themselves together despite the repulsion due to their same charge
Describe the 3rd rule when deciding electronic configuration
Rule 3 : Hund’s Rule of Multiplicity
WHen a number of orbitals of equal energy are available, electrons occupy them singly first before any pairing occurs
Electrons prefer to occupy separate orbitals within the same subshell
Pairing only occurs after all degenerate orbitals in a subshell are singly occupied
State the electronic configuration of Cr and Cu and why
Electronic configuration of Cr : 1s2 2s2 2p6 3s2 3p6 3d5 4s1 (NOT 1s2 2s2 2p6 3s2 3p6 3d4 4s2)
Electronic configuration of Cu : 1s2 2s2 2p6 3s2 3p6 3d10 4s1 (NOT 1s2 2s2 2p6 3s2 3p6 3d9 4s2)
Reason : Half-filled and fully-filled 3d orbitals (3d5, 3d10) are more stability
Cr : As 4s and 3d orbitals are close in energy, the electronic configuration with half-filled 3d subshell is relatively stable as it minimises electrostatic repulsion
Cu : Due to stability of full 3d subshell
Define transition element
Transition element (Def.) : A transition metal is a d-block element that form one or more stable ions with a partially-filled d-subshell
Describe the trend in atomic radius down the group
General trend : Increases down the group Explanation : The number of quantum shells increases the outermost electrons are further away from the nucleus, hence the atomic radius increases. Both the nuclear charge and shielding effect increase down the group, hence the effective nuclear charge differs little down the group |
Describe the trend in atomic radius across periods 2 and 3
General trend : decreases down the group
Explanation :
nuclear charge increases due to the increase in the number of protons in the nucleus
shielding effect remains relatively constant (as the electrons are added to the same outermost shell)
effective nuclear charge increases -> stronger electrostatic forces of attraction between the nucleus and the outermost electron
outermost electrons are pulled closer to the nucleus and hence a decrease in the atomic radius
Describe the trend in atomic radius across 1st row transition elements
General trend : relatively invariant Explanation :
|
Describe the trend in ionic radius of cations and anions
Ionic radius of cations: Na+ > Mg2+ > Al3+ > Si4+
Ionic radius of anions:P3 > S2 > Cl−
Isoelectronic : same number of electrons
Explanation :
Across the two isoelectronic series (Na+ to Si4+ and P3− to Cl−), nuclear charge increases and the shielding effect is the same (due to the same number of electrons).
This results in an increase in the effective nuclear charge and a stronger attraction between the outermost electrons and the nucleus, hence a decrease in ionic size across each series.
There is a sharp increase in ionic radius from the cationic series of Na+ to Si4+ to the anionic series of P3 to Cl, because the anions have one more quantum shell of electrons than the cations
Define ionisation energy
N ionisation energy (Def.) : Energy required to remove 1 mole of electrons from 1 mole of gaseous ions with (n1)+ charge to form 1 mole of gaseous ions with n+ charge
Define 1st and 2nd ionisation energy
1st ionisation energy (Def.) : Energy needed to remove 1 mole of electrons from 1 mole of gaseous atoms to form 1 mole of gaseous ions with a single positive charge
2nd ionisation energy (Def.) : Energy required to remove 1 mole of electrons from 1 mole of uni positively charged gaseous ions to form 1 mole of gaseous ions with double positive charge
Explain why ionisation energies become more endothermic
As successive electrons are removed, the number of protons remains unchanged (nuclear charge remains constant). With fewer electrons present, the shielding effect is lower. Effective nuclear charge experienced by the remaining electrons increases, leading to a stronger attraction between the nucleus and the electrons -> more energy is required to remove the successive electrons.
Describe effective nuclear charge
Net nuclear charge experienced by an outer electron
Dependent on :
Nuclear charge (Z) : The nuclear charge is dependent on the number of protons. The larger the number of protons, the greater the nuclear charge and the attraction for the outermost electrons by the nucleus
Shielding or screening effect (S) : Reduction of Zeff due to repulsion from other electrons. Inner electrons “shield” outer electrons from full attraction force of positively charged nucleus
Zeff = Z – S
Describe and explain the factors of ionisation energy
Number of quantum shells
The larger the number of quantum shells in an atom, the further the electron is from the nucleus and hence experiences weaker nuclear attraction and is easier to remove, hence the lower the ionisation energy
Effective nuclear charge, Zeff
Nuclear charge effect
Total positive charge of all the protons in the nucleus
Increases with increasing proton number
Shielding effect
Shielding electrons are inner shell electrons found in between nucleus and valence electrons
They cause shielding effect which reduces strength of nuclear attraction experienced by valence electrons
Number of inner shell electrons increase -> shielding effect increases
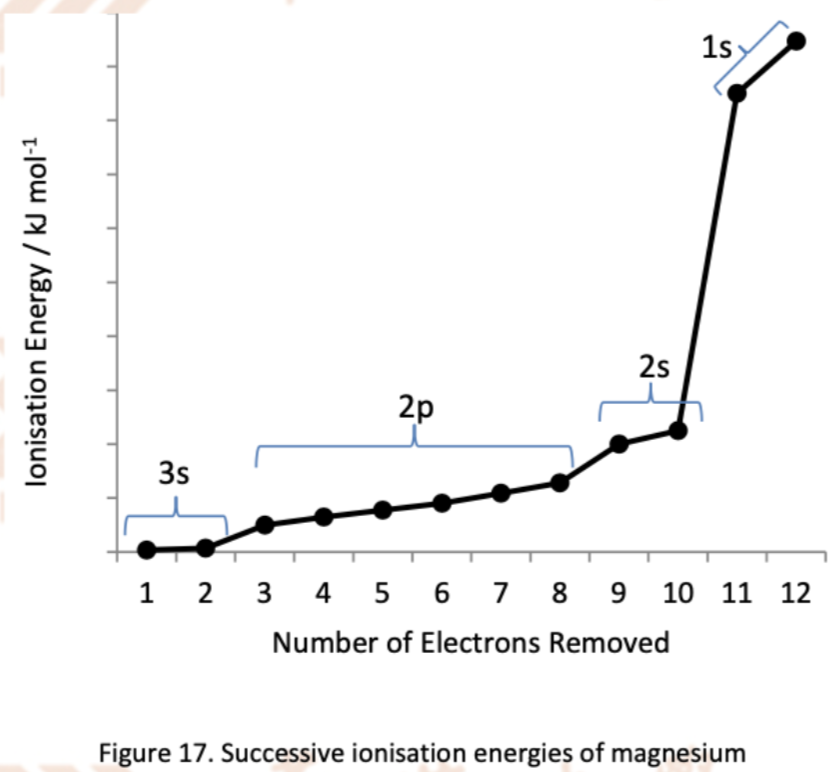
Explain the trend in ionisation energy of this element
The first big increase from the 2nd to 3rd ionisation energies (when 3rd electron is removed)
Implies that the third electron is in an inner quantum shell, and thus there are two electrons in the outermost or valence 3s subshell
Conclusion: Mg is a Group 2 element
The gradual increase from the 3rd to 10th ionisation energies
Electrons 3 and 4 are removed from the same subshell within the same shell -> close in energies -> small difference in energy observed
A slightly greater increase from the 8th to 9th ionisation energies : 8th is removed from 2p and 9th is removed from 2s -> 2s subshell is closer to nucleus and is lower in energy than 2p subshell -> larger amount of energy is required to remove the 2s electrons
The second big increase from the 10th to 11th ionisation energies
Implies that the last two electrons are in the first (innermost) quantum shell
Describe the trend in ionisation energy down the group
General : 1st IE decreases down the group Explanation : Down the group, the number of inner shells of electrons increases, hence the outermost electrons are further from the nucleus. Therefore, the electrostatic forces of attraction between the nucleus and the outermost electron is weaker and less energy is required to remove this electron *Nuclear charge increases, effect is cancelled out by increase in shielding effect due to increase in inner electrons |
Describe the trend in ionisation energy across period 2 and 3
General trend : 1st IE increases across a period
Explanation : Across the period, nuclear charge increases and the shielding effect remains relatively constant as the number of inner shell electrons remains the same and electrons are being added to valence electron shell. Hence the effective nuclear charge increases and the electrostatic forces of attraction between the outermost electrons and the nucleus become stronger, so more energy is required to remove the outermost electron.
Describe the anormalies in period 2 and 3 IE trends
Small dip between Group 2 and Group 13 elements
Al has lower 1st ionisation energy than Mg.
Mg: 1s2 2s2 2p6 3s2 Al: 1s2 2s2 2p6 3s2 3p1
The 3p subshell of Al is further away from the nucleus than the 3s subshell
There is a weaker attraction between the nucleus and the outermost electron -> less energy is required to remove the 3p electron from Al
Small dip between Group 15 and Group 16 elements
S has lower 1st IE than P
All the 3p electrons in P are unpaired. In S, one of the 3p electrons are paired
There is some interelectronic repulsion between the paired electrons in the 3p subshell in S -> less energy than expected is required to remove one of these paired electrons from S
Describe the trend in ionisation energies across transition elements
General trend : remains relatively invariant Explanation : (same as atomic radius one)
|