Orgo 2 Test 2 reactions
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
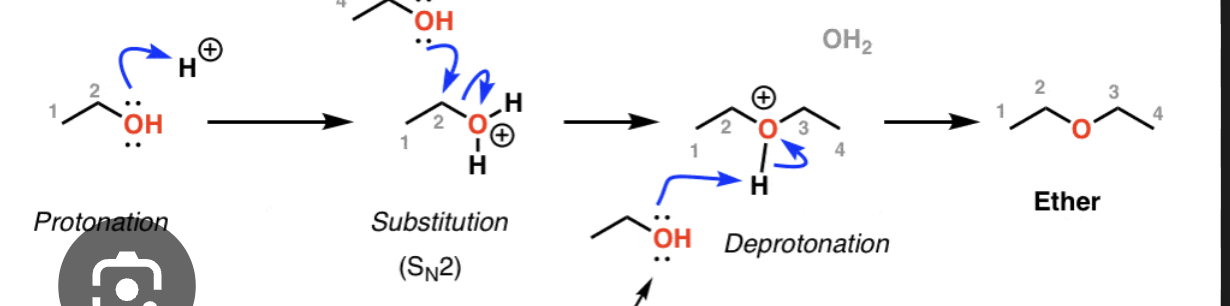
Acid Catalyzed condesation of alchohols (+ mech)
Prepare ethers by adding alkoxides to primary or methyl alkyl halides. (Williamson ether synthesis)
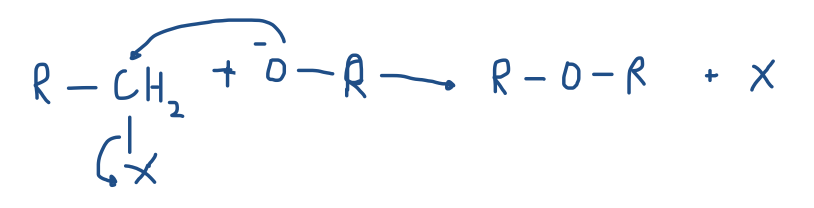
Alkoxymeruration alkene
acid-catalyzed cleavage of ethers by excess HBr or HI
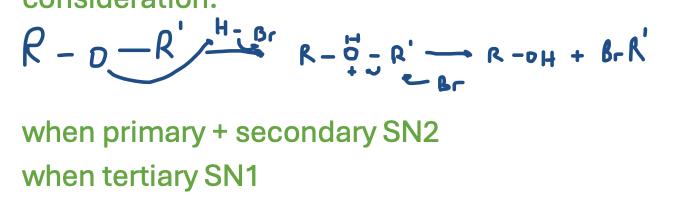
Claisen Rearrangement
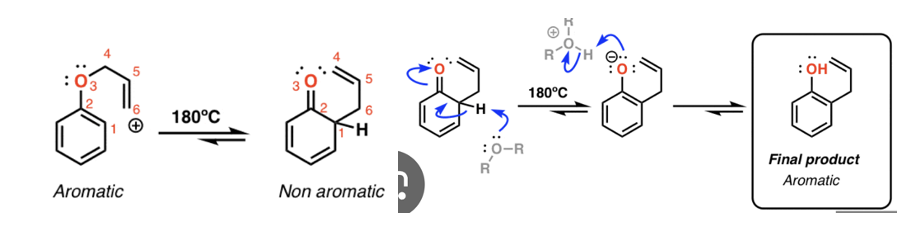
Alkene with peroxyacid (mCBPA).
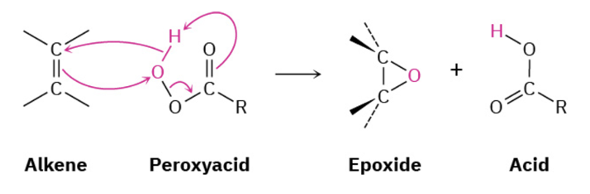
Provide products and mechanisms for the reaction of vicinal halohydrins in base to form epoxides
Provide products for nucleophilic ring-opening reactions of epoxides involving either anionic nucleophiles or acid-catalyzed reactions of neutral nucleophiles.
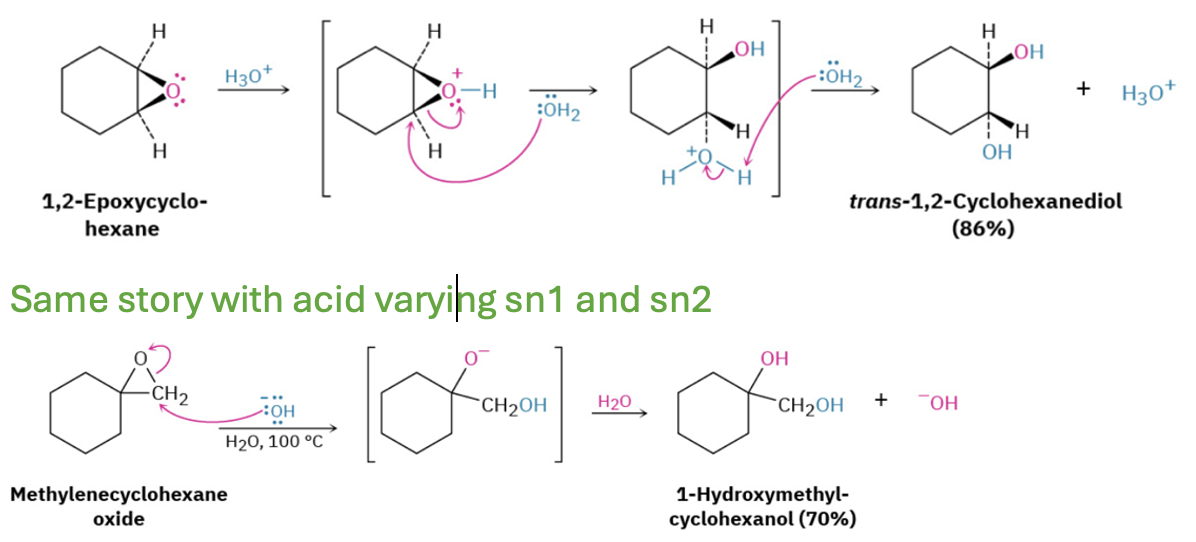
Provide missing reagents, products, and a mechanism for the synthesis of thiols by the alkylation and hydrolysis of thiourea.
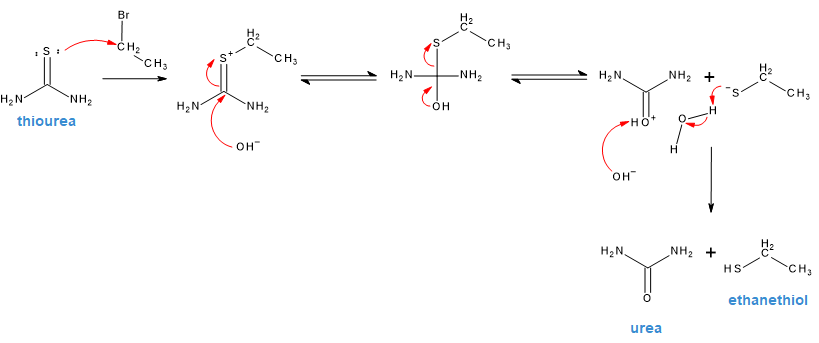
Provide products of the reaction between an alkane thiolate and an alkyl halide. (SN2)
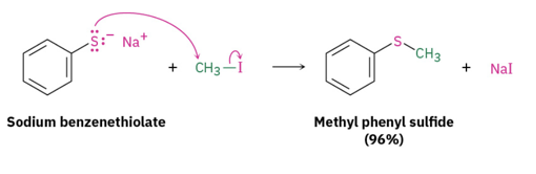
Provide products or missing reagents for the oxidation of thiols to disulfides using iodine and the reverse reduction using sodium borohydride or zinc/acid.
Synthesize aldehydes from primary alcohols, from alkenes, or from esters
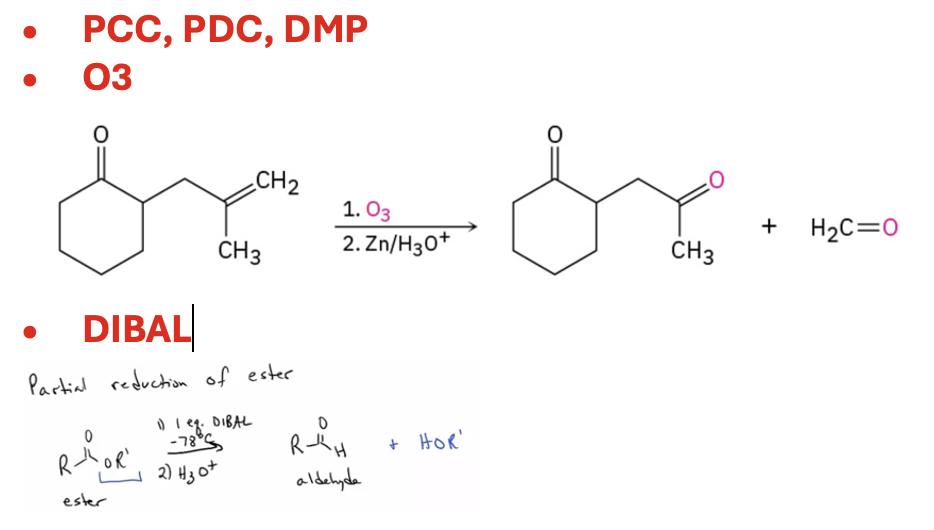
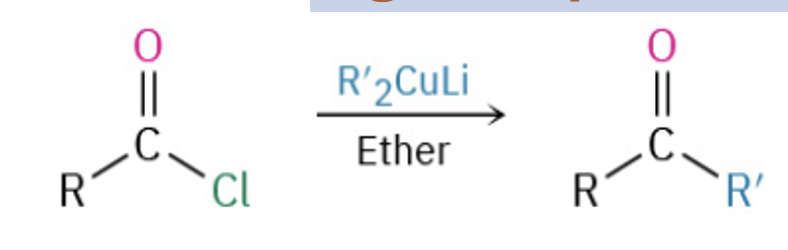
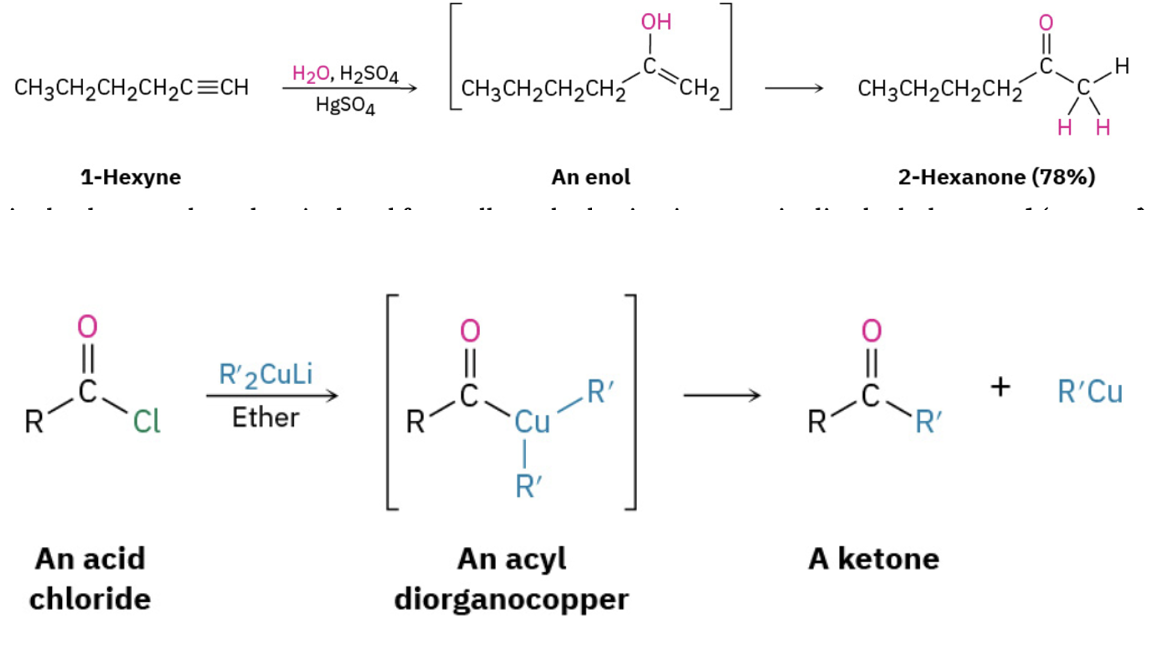
Synthesize ketones by oxidation of secondary alcohols, from the ozonation of alkenes, by addition of water to an alkyne , or from the reaction of an acid chloride and a cuprate
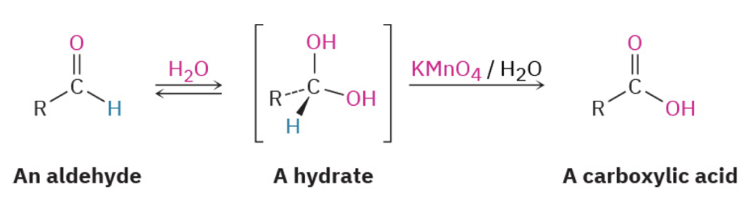
Provide products or missing reagents (but not a mechanism) for the oxidation of aldehydes to carboxylic acids.
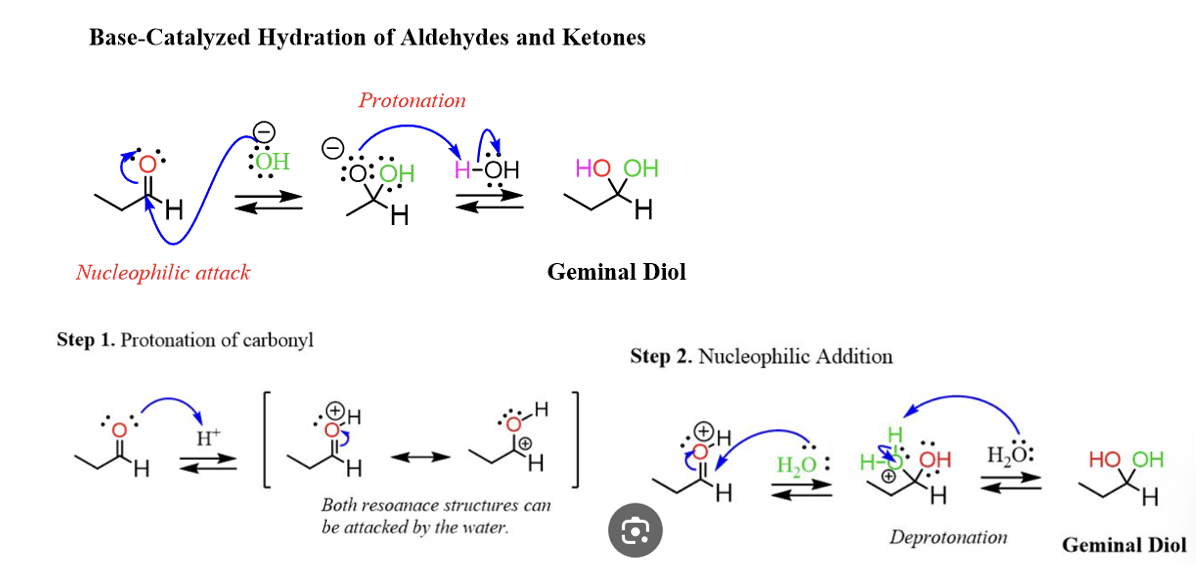
Provide products and mechanism for hydration of aldehydes and ketones under either acidic or basic conditions
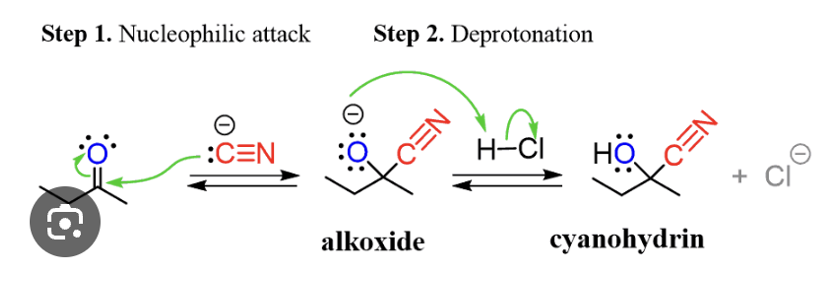
Provide products and a mechanism for the addition of HCN to an aldehyde or ketone to form a cyanohydrin
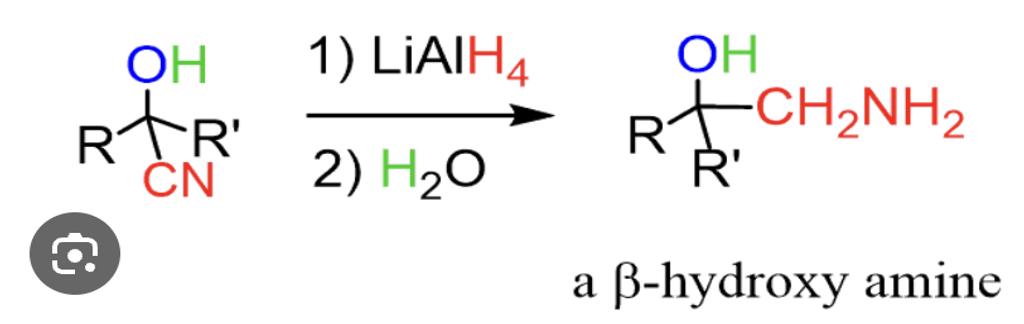
Provide products for the reduction of a cyanohydrin to yield a vicinal aminoalcohol.
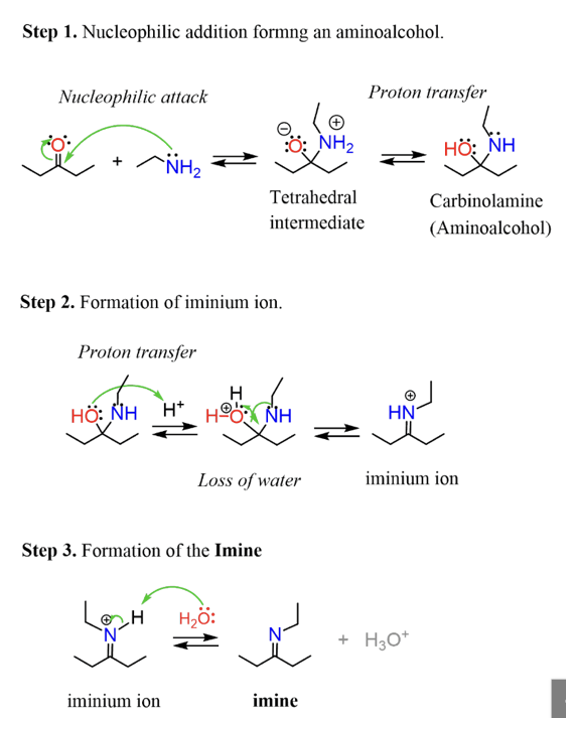
Provide products and mechanism for the formation of imines from aldehyde or ketone plus primary amine.
(NO BASE)
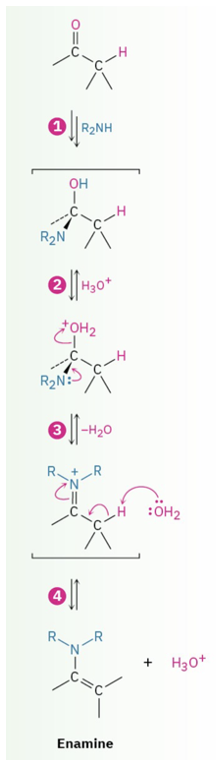
Provide a product and mechanism for the formation of enamines from aldehyde or ketone plus secondary amine.
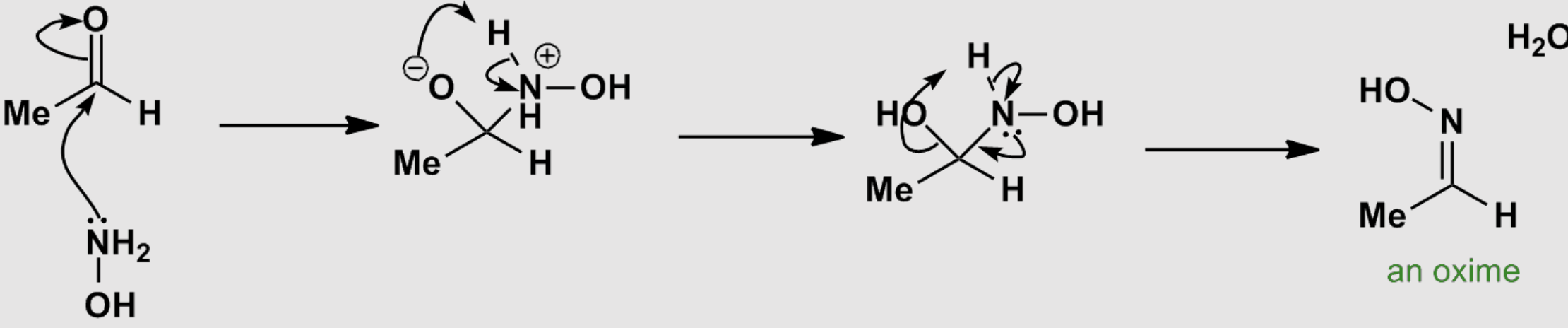
aldehydes and ketones with
hydroxylamine
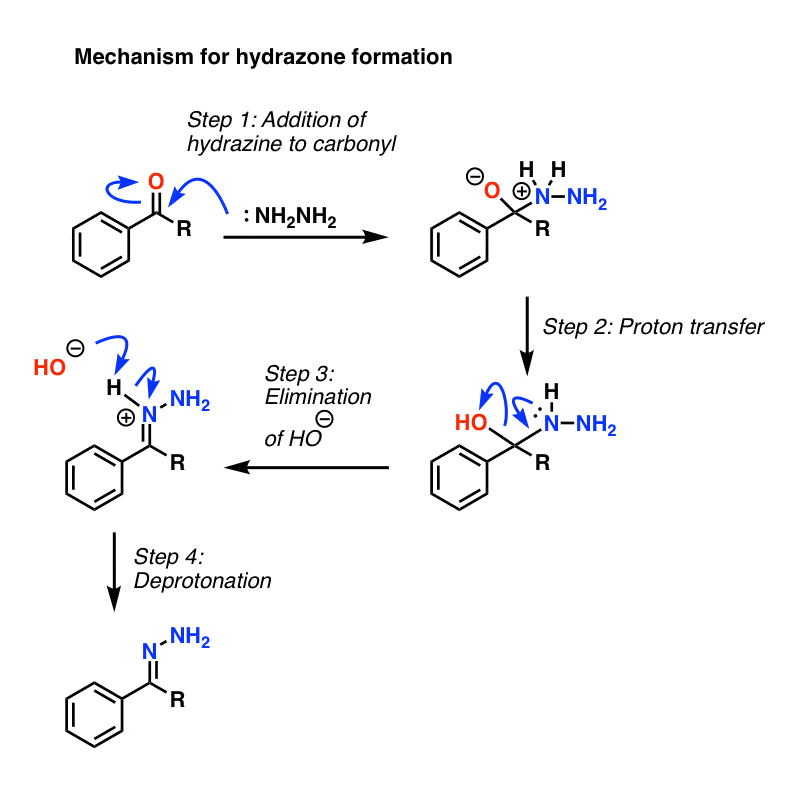
aldehydes and ketones with
hydazine
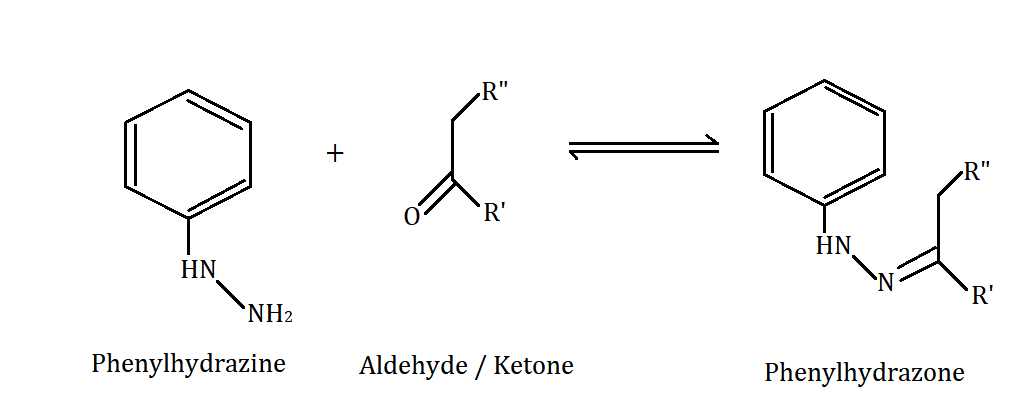
aldehydes and ketones with
phenolhydrazine
aldehydes and ketones with
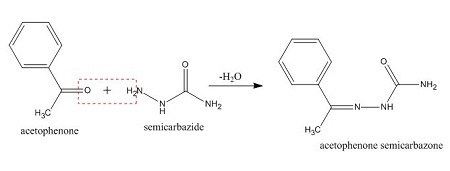
semicarbazide
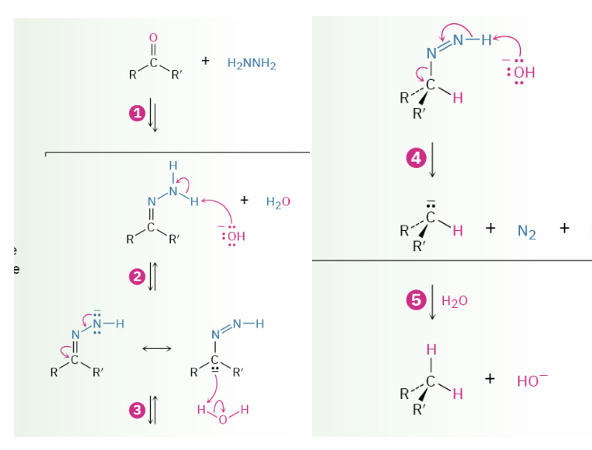
Wolff-Kishner
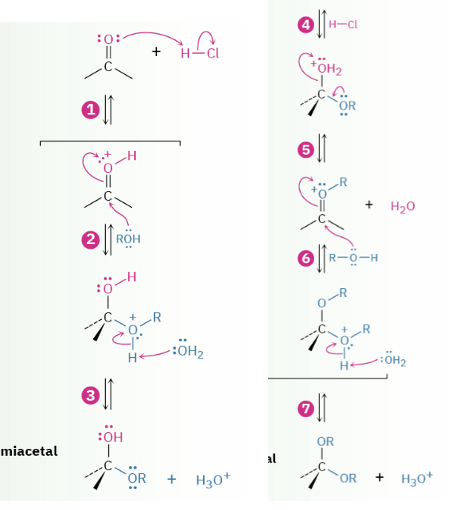
Provide products and mechanism for the acid-catalyzed formation of acetals (from aldehydes or ketones.)
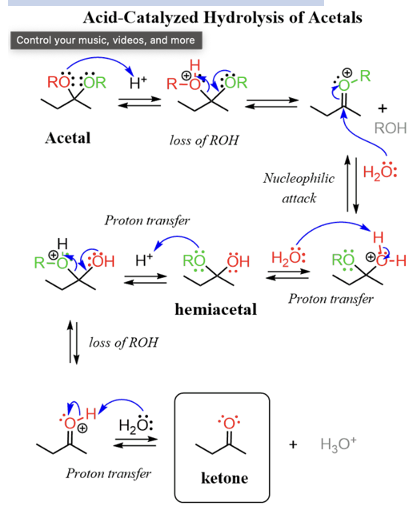
Provide products and mechanisms for the conversion of acetals to aldehydes or ketones
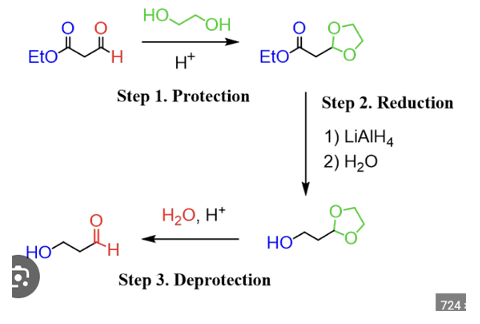
Use acetals as protecting groups for synthetic schemes
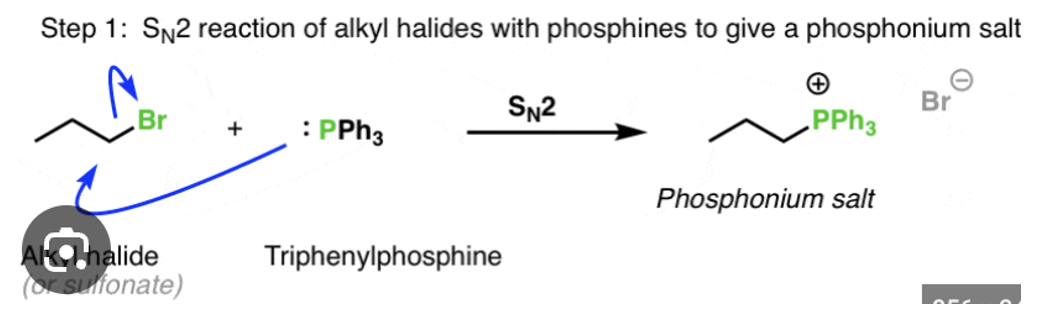
Synthesize phosphorus ylides from alkyl halides (NBuLi to make it + and -)
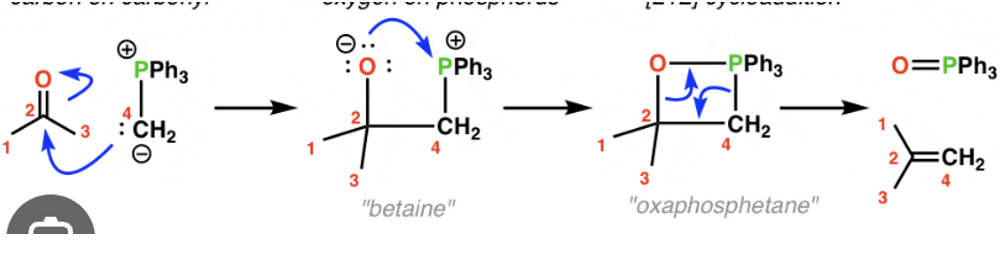
Synthesize alkenes using the Wittig reaction of a phosphorus ylide with an aldehyde or ketone.
carbox acid by oxidation primary alc
Strong oxidizer KMNO4 or H2CrO4
carbox by oxididation aldehyde
Na2Cr2O7/H2SO4, KMnO4, CrO4, Tollens’ (Ag), Benedict Reagent (Cu) specifically for aldehyde to carboxylic acid, won’t do OH
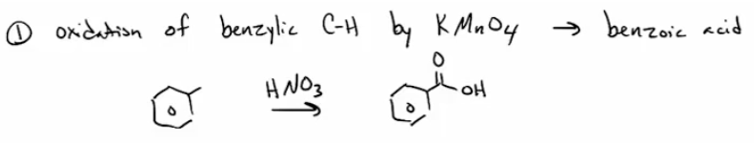
Cabrox by ox of benzene C-H
reaction of Grignard, organolithium, or organosodium reagents with CO2

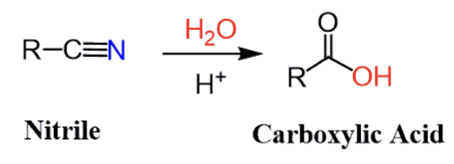
acid catalyzed hydrolysis of a nitrile.
Ozonation of an alkene followed by an oxidative workup will yield ___

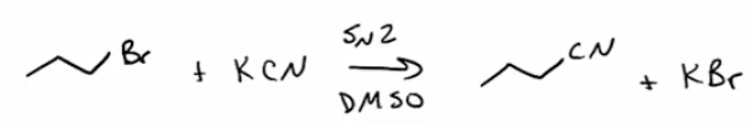
Provide a synthesis and mechanism (SN2) for nitriles from methyl or primary alkyl halides + KCN.
Provide products or missing reagents for the dehydration of amides to nitriles upon addition of SOCl2 or P4O10 or POCl3.
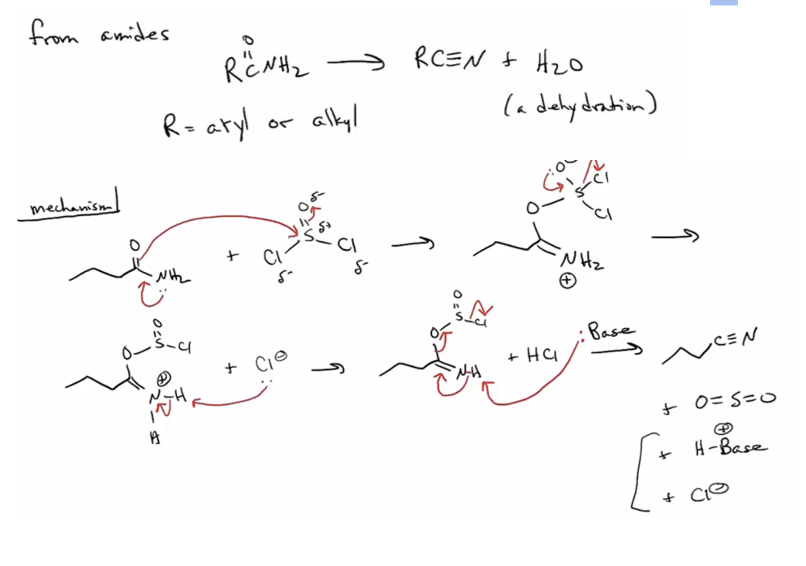
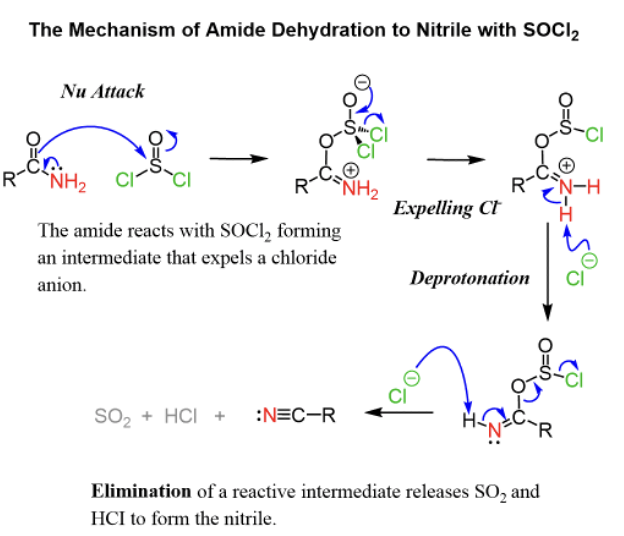
Provide products, missing reagents, or a mechanism for the dehydration of primary amides to nitriles upon addition of SOCl2.
Provide products and a mechanism for the reaction of a nitrile with a Grignard followed by an aqueous acidic workup
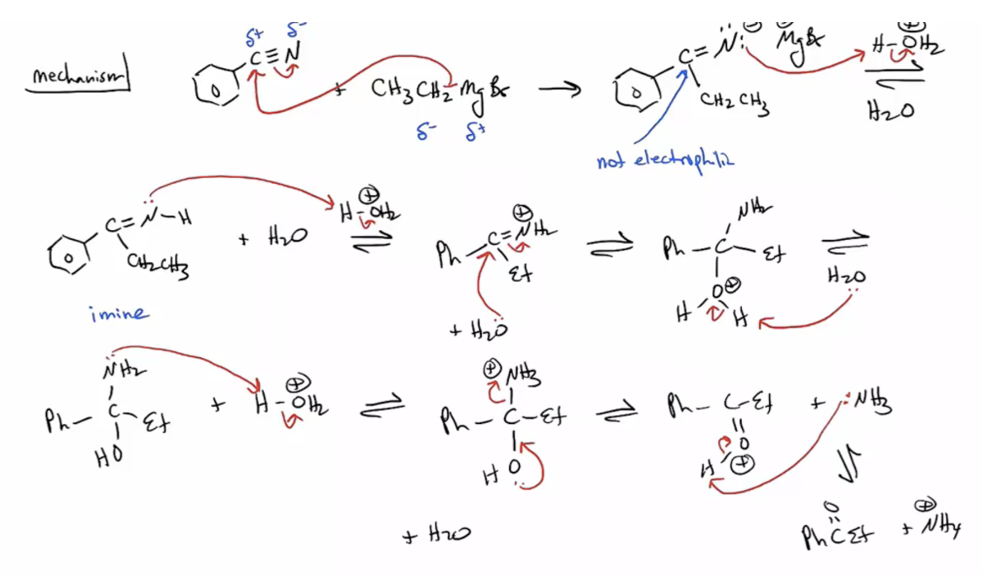
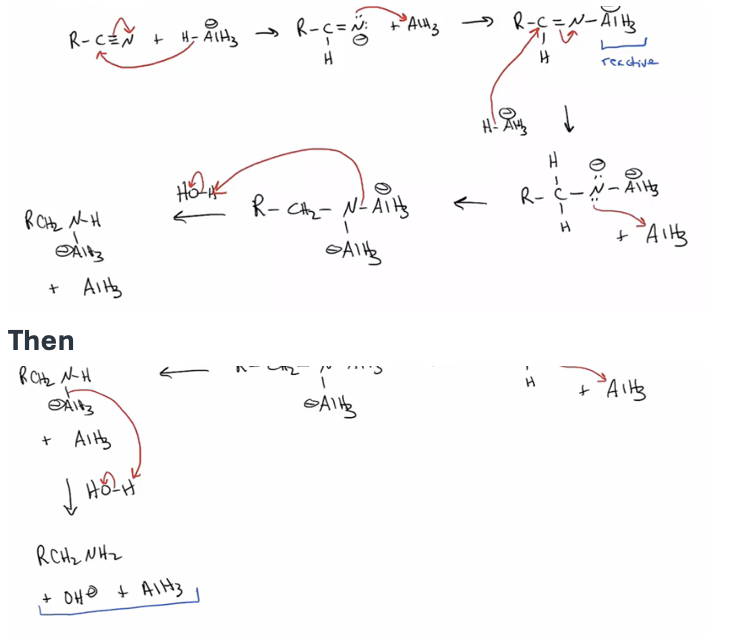
Provide products and a mechanism for the reduction of nitriles by the addition of LiAlH4.
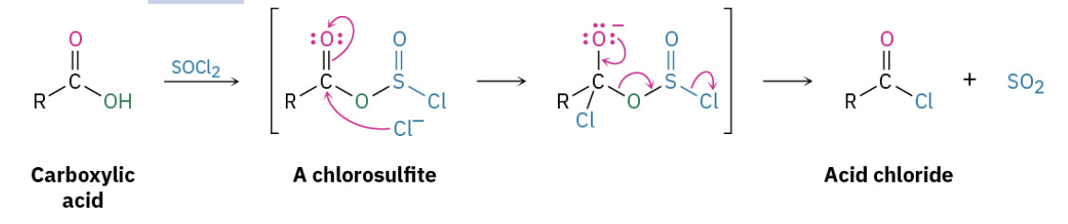
carboxylic acids and SOCl2 or PCl3. (21.3)
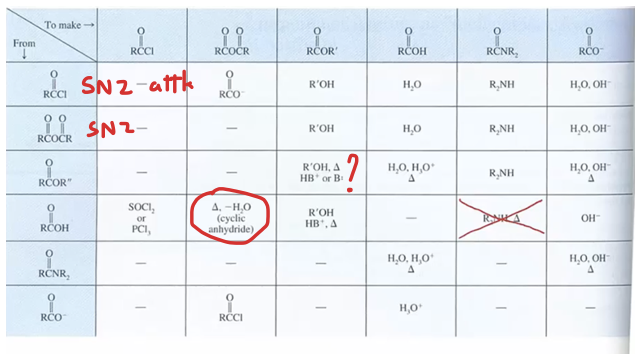
Interconvert between the carboxylic acid derivatives (table)
Provide products and a mechanism for the conversion of carboxylic acids + amines to amides using DCC.
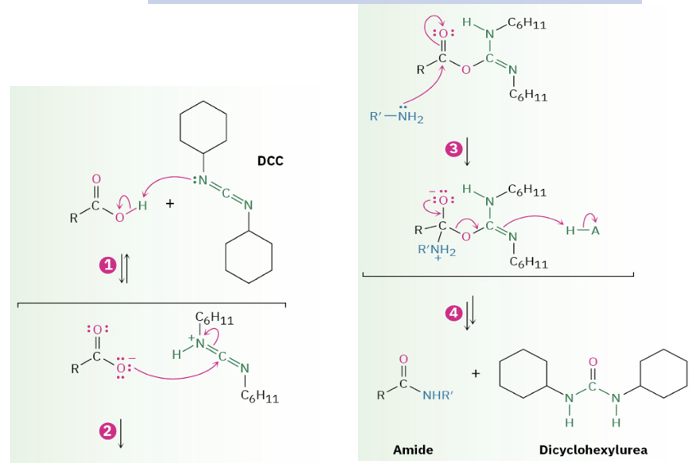
Provide complete electron-pushing mechanisms for any of the carboxylic acid derivative conversions.
show it
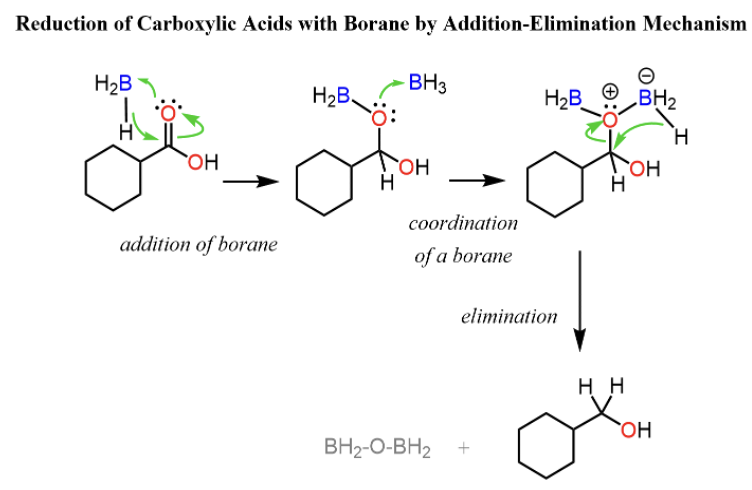
Provide products or missing reagents for the reduction of carboxylic acids to _____upon addition of BH3. (the fastest reducer)
Provide products and a mechanism for the reaction of 2 equivalents of organolithium, organosodium, or Grignard reagents to an acid chloride
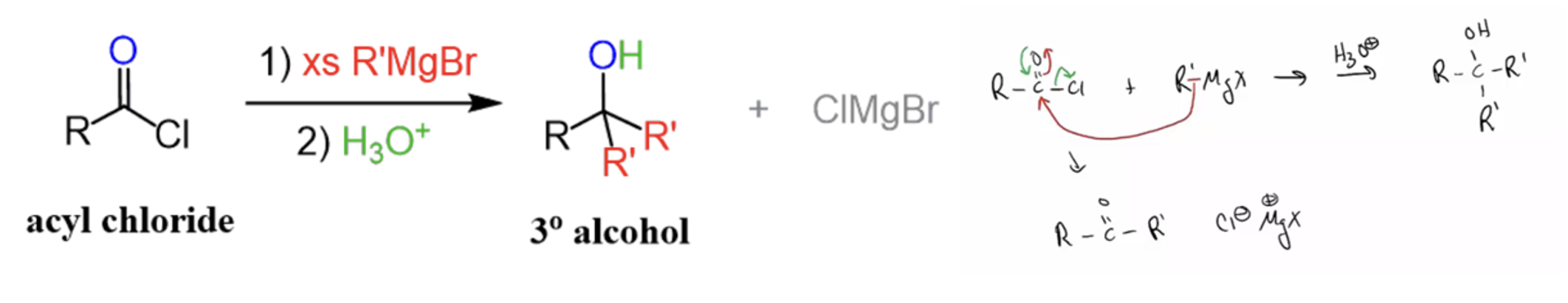
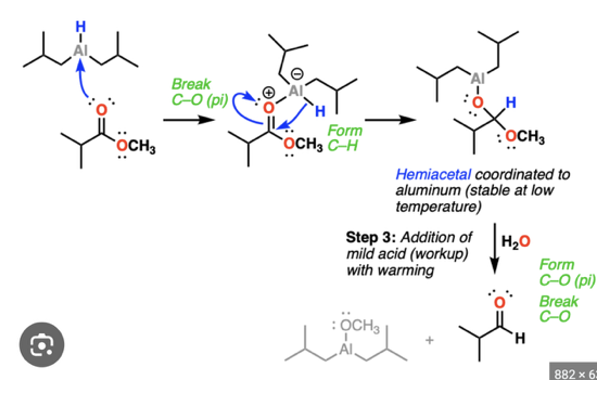
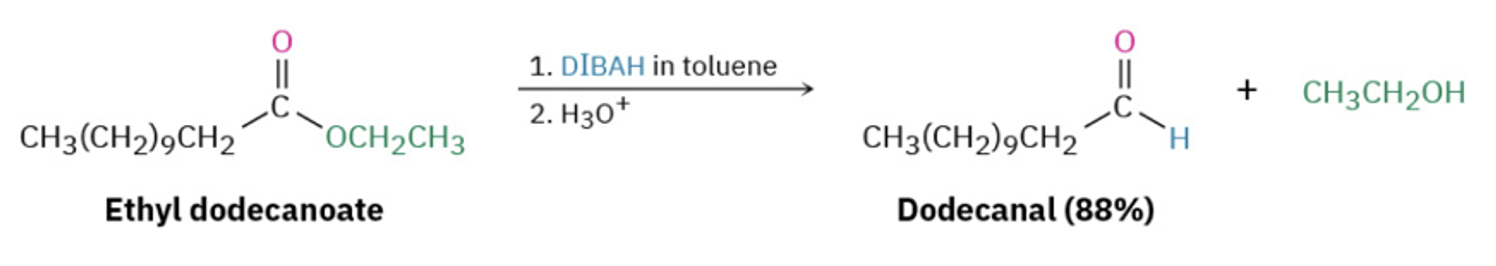
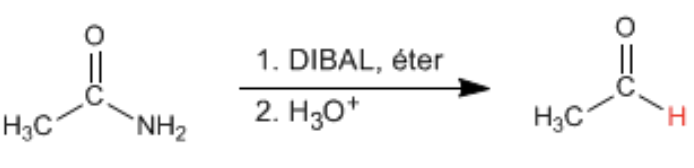
Provide the products of the reaction of 1 eq. DIBAL (= DIBAH), cold, with an ester AND to an amide
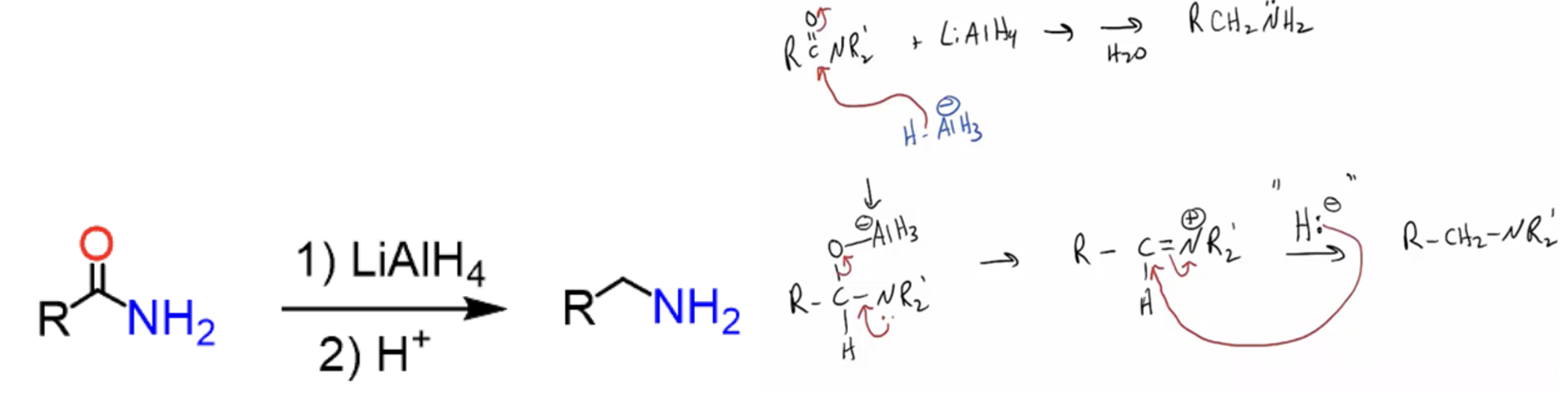
Provide products and a mechanism for the reduction of amides by LiAlH4. (C=O becomes CH2.)
Provide the products (but not a mechanism) for the addition of an organocuprate to acid chloride