Bio- Water, and Carbs(A1.1, B1.1)
5.0(2)
Card Sorting
1/66
There's no tags or description
Looks like no tags are added yet.
Last updated 1:19 PM on 9/1/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
67 Terms
1
New cards
water is the medium for ____
metabolic reactions
2
New cards
water is a ____ medium
transport
3
New cards
what does two water molecules bonded together look like?
(don’t forget the weird ‘s’ before the + or -)
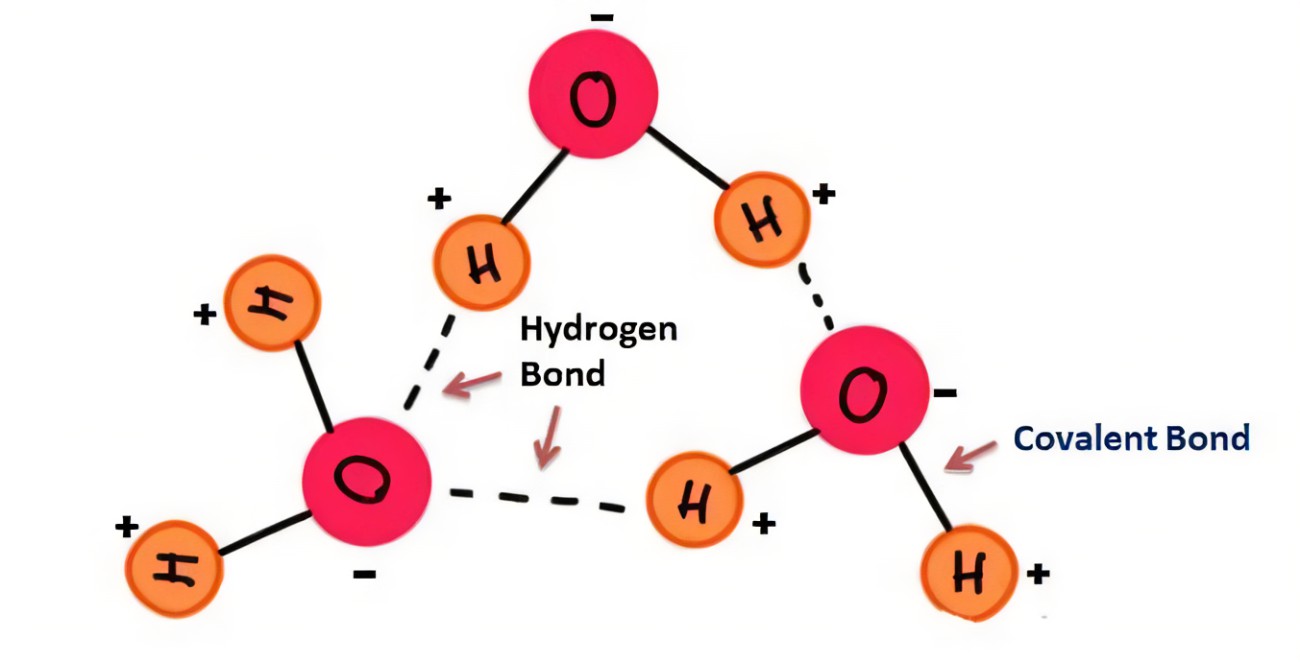
4
New cards
Cohesion among water molecules is primarily due to what?
hydrogen bonding
5
New cards
water can move through a cell wall primarily due to what?
hydrogen bonds between water molecules and the cell wall (adhesion)
6
New cards
hydrogen bonding between water molecules is responsible for what?
high surface tension
7
New cards
how do polar covalent bonds in water molecules give rise to the formation of hydrogen bonds?
polar covalent bonds in water give rise to the formation of hydrogen bonds because the slightly negative charge of oxygen is attracted to the slightly positive charge of hydrogen.
8
New cards
what is an example of why hydrogen bonding is important?
transport in the xylem, cohesion between the water molecules (the strong hydrogen bonds) pulls the water up from the roots to the leaves (adhesion when connecting to the cell wall to get pulled up)
9
New cards
what is surface tension?
property of a substance to resist an external force
10
New cards
how does surface tension occur?
cohesive nature of the molecules (water has a stronger pull to each other than they do to the air)
11
New cards
what is an example of surface tension?
water striders are insects that walk on water. because the water molecules have a stronger pull to each other than the insect, the bug can walk on water easily, creating a habitat.
12
New cards
what is capillary action?
the ability of water to flow against gravity in a narrow space
13
New cards
how does capillary action occur in soil?
water adheres to the polar soil particles and moves up the soil towards the roots of the plants (greater in fine clay soils and weaker in porous sandy soils)
14
New cards
how does capillary action occur in the cell wall?
water adheres to cellulose molecules in cell walls, if water evaporates from the cell walls and is lost to the atmosphere, adhesive forces cause water to be drawn out of the nearest xylem vessel (low pressure on inside against the atmospheric high pressure, high pressure at bottom and low pressure at top)
15
New cards
what are hydrophilic substances?
charged substances that mix and dissolve with water
16
New cards
why do a lot of hydrophilic substances dissolve in water?
because of water’s polarity
17
New cards
when do most metabolic reactions occur?
when the reactants are dissolved in water
18
New cards
how does water transport in plants?
polar molecules like sucrose and amino acids dissolve in water and are transported through sap in the phloem of plants (because of hydrogen bonding)
19
New cards
why do ions dissolve in water?
the polar water molecules surround the charged ions allowing transport in the xylem sap
20
New cards
how does water transport in animals?
animals transport many things through the blood or hemolymph (both have a base of water) like nutrients, oxygen, carbon dioxide, hormones, waste products of metabolism (poo or pee), antibodies, and/or heat
21
New cards
what are the modes of transport in blood?
glucose, amino acids, and sodium chloride- dissolved in blood plasma. cholesterol and lipids- lipoprotein complexes. oxygen- mostly attached to hemoglobin but can dissolve in water sometimes (not well) (dissolved oxygen)
22
New cards
what is metabolism?
all the chemical reactions occurring in living organisms
23
New cards
what are enzymes?
biological catalysts that speed up the rate of chemical reactions (involved in controlling metabolism)
24
New cards
where do metabolic reactions occur?
in aqueous solutions with the reactants and enzymes dissolved in water
25
New cards
does water dissolve hydrophobic substances?
no. hydrophobic substances are not charged and do not readily mix with water
26
New cards
what are the functions of hydrophobic molecules?
some molecules in living creatures are hydrophobic and do not dissolve in water, the functions of these substances depend on them being hydrophobic
27
New cards
what are some examples of hydrophobic substances?
fats (triglycerides) are hydrophobic (entirely nonpolar). to prevent them from coalescing and forming large droplets in blood, small droplets are coated with a single layer of phospholipids (amphipathic molecule), allowing fay droplets to real suspended in blood plasma
28
New cards
what is buoyancy?
the upward force exerted by a fluid on an object immersed in the fluid
29
New cards
what is denser than air?
liquid water is a denser fluid than air, providing greater buoyancy for aquatic animals and allowing them to float or swim more easily
30
New cards
contrast the buoyancy of water with that of air with the black-throated loon and the ringed seal as examples
* water is more dense than air so less energy is required for the seal to float in water
* air is less dense than water so the loon spends more energy to fly
* does floating for the seal or flying for the loon require more energy?
* flying for the loon because it is less dense / less buoyant
* air is less dense than water so the loon spends more energy to fly
* does floating for the seal or flying for the loon require more energy?
* flying for the loon because it is less dense / less buoyant
31
New cards
what is viscosity?
the measure of a fluids resistance to flow (the higher the viscosity, the more difficult it is for animals to move through the fluid)
32
New cards
what has a higher viscosity than air?
water has a higher viscosity than air, and many aquatic animals (the black throated loon) have a streamlined body shape (adaptations) which allows them to smoothly move through water
33
New cards
contrast the viscosity of water with that of air using the black throated loon and ringed seal as examples
* water is more viscous than air so more energy is required for the seal to move through the water
* air is less viscous than water so less energy is required for the loon to move through the air
* does swimming through water for the seal or flying through air for the loon require more energy?
* swimming through the water for the seal
* air is less viscous than water so less energy is required for the loon to move through the air
* does swimming through water for the seal or flying through air for the loon require more energy?
* swimming through the water for the seal
34
New cards
what is thermal conductivity?
the rate at which heat passes through a material (heat always moves from a higher temperature to a lower temperature)
35
New cards
what has a higher thermal conductivity than air?
water has a higher thermal conductivity than air. aquatic animals are more likely to lose heat to the environment and must be adapted to reduce heat loss (lose heat faster in water)
36
New cards
what are some examples of thermal conductivity?
* ringed seals have a layer of insulating blubber to prevent heat loss in the water
* seals will huddle together out of the water to reduce heat lose by decreasing the surface area in contact with the air
* black throated loons are insulated by feathers which are coated with a hydrophobic oil to keep the feathers dry, which reduces heat loss
* seals will huddle together out of the water to reduce heat lose by decreasing the surface area in contact with the air
* black throated loons are insulated by feathers which are coated with a hydrophobic oil to keep the feathers dry, which reduces heat loss
37
New cards
contrast the thermal conductivity of water with that of air using the black throated loon and ringed seal as examples
* water has a higher thermal conductivity than air so the ringed seals have insulating blubber and their huddling together to slow thermal conductivity in the water and on land
* air has a lower thermal conductivity than water so the black throated loon has hydrophobic feathers to slow thermal conductivity in water and on land
* air has a lower thermal conductivity than water so the black throated loon has hydrophobic feathers to slow thermal conductivity in water and on land
38
New cards
what is specific heat capacity?
the energy required to raise the temperature of 1g of a substance by 1K (or 1C, Celsius)
39
New cards
what has a higher specific heat capacity than air?
water has a much higher specific heat capacity than air. water has a very high specific heat because it takes a lot of energy to break the hydrogen bonds between water molecules, changing the temperature of water (has a stable temperature)
40
New cards
how does specific heat capacity apply to water vs animals?
* water
* temperature of large bodies of water is more stable than air, as the water can absorb or release much more heat energy without experiencing significant temperature change
* animals
* the cells (which are mostly comprised of water) are also resistant to temperature change, which helps maintain constant body temperatures in endotherms like birds or animals (enzymes function in a narrow range of temperature so if the temperature is too high for too long, you can die because the enzymes cannot work properly)
* temperature of large bodies of water is more stable than air, as the water can absorb or release much more heat energy without experiencing significant temperature change
* animals
* the cells (which are mostly comprised of water) are also resistant to temperature change, which helps maintain constant body temperatures in endotherms like birds or animals (enzymes function in a narrow range of temperature so if the temperature is too high for too long, you can die because the enzymes cannot work properly)
41
New cards
contrast the specific heat capacity of water with that of air
* water has a higher specific heat capacity than air
* aquatic habitats are more thermally stable than terrestrial habitats because it wakes more energy to change temperatures in water
* air has a lower specific heat capacity than water
* it helps keep the body’s temperature constant making it a comfortable place for enzymes to properly function well
* aquatic habitats are more thermally stable than terrestrial habitats because it wakes more energy to change temperatures in water
* air has a lower specific heat capacity than water
* it helps keep the body’s temperature constant making it a comfortable place for enzymes to properly function well
42
New cards
what is the extraplanetary origin of water (and reason for retention)? (where did it come from and why did it stay here?)
* 98% of water on earth is liquid and has allowed for life to evolve.
* the most widely supported hypothesis of the origin of water is this:
* heavy bombardment of water-filled asteroids hit earth during the first few hundred years of formation.
* water has stayed on earth because of two reasons:
* gravity (can hold the water on earth)
* the distance of earth from the sun
\
* the most widely supported hypothesis of the origin of water is this:
* heavy bombardment of water-filled asteroids hit earth during the first few hundred years of formation.
* water has stayed on earth because of two reasons:
* gravity (can hold the water on earth)
* the distance of earth from the sun
\
43
New cards
what is the Goldilocks zone?
when the planet is not too hot and not too cold for liquid water to exist. the conditions must be “just right for the liquid water”
44
New cards
what are the macromolecules that carbon can form
carbohydrates, lipids, proteins, and nucleic acids
45
New cards
how many valence electrons does carbon have?
4 valence electrons allowing it to form up to 4 covalent bonds with other carbon atoms or other non-metal elements. the bonds can be single bonds or a combination of single and double bonds (covalent bonds are bonds shared between two non-metals)
46
New cards
what kinds of shapes can carbon compounds take?
* unbranched chains (fatty acids)
* branched chains
* single rings (glucose)
* multiple rings (cholesterol)
* branched chains
* single rings (glucose)
* multiple rings (cholesterol)
47
New cards
what are the monomers, polymers, and macromolecules
* glucose - polysaccharide - amylose and amylopectin in starch, cellulose
* amino acid - polypeptide - proteins
* nucleotide - polynucleotide - DNA and RNA
* fatty acids and glycerol - triglyceride - fats and oils
* amino acid - polypeptide - proteins
* nucleotide - polynucleotide - DNA and RNA
* fatty acids and glycerol - triglyceride - fats and oils
48
New cards
what is a condensation reaction?
it combines two molecules releasing a water molecule
49
New cards
what is a hydrolysis reaction?
it involves the addition of a water molecule to hydrolyze (break down) a large molecule
50
New cards
how do you differentiate between a pentose sugar or a hexose sugar?
you count the amount of carbons the molecule has starting on the right after the oxygen
51
New cards
what does glucose look like?
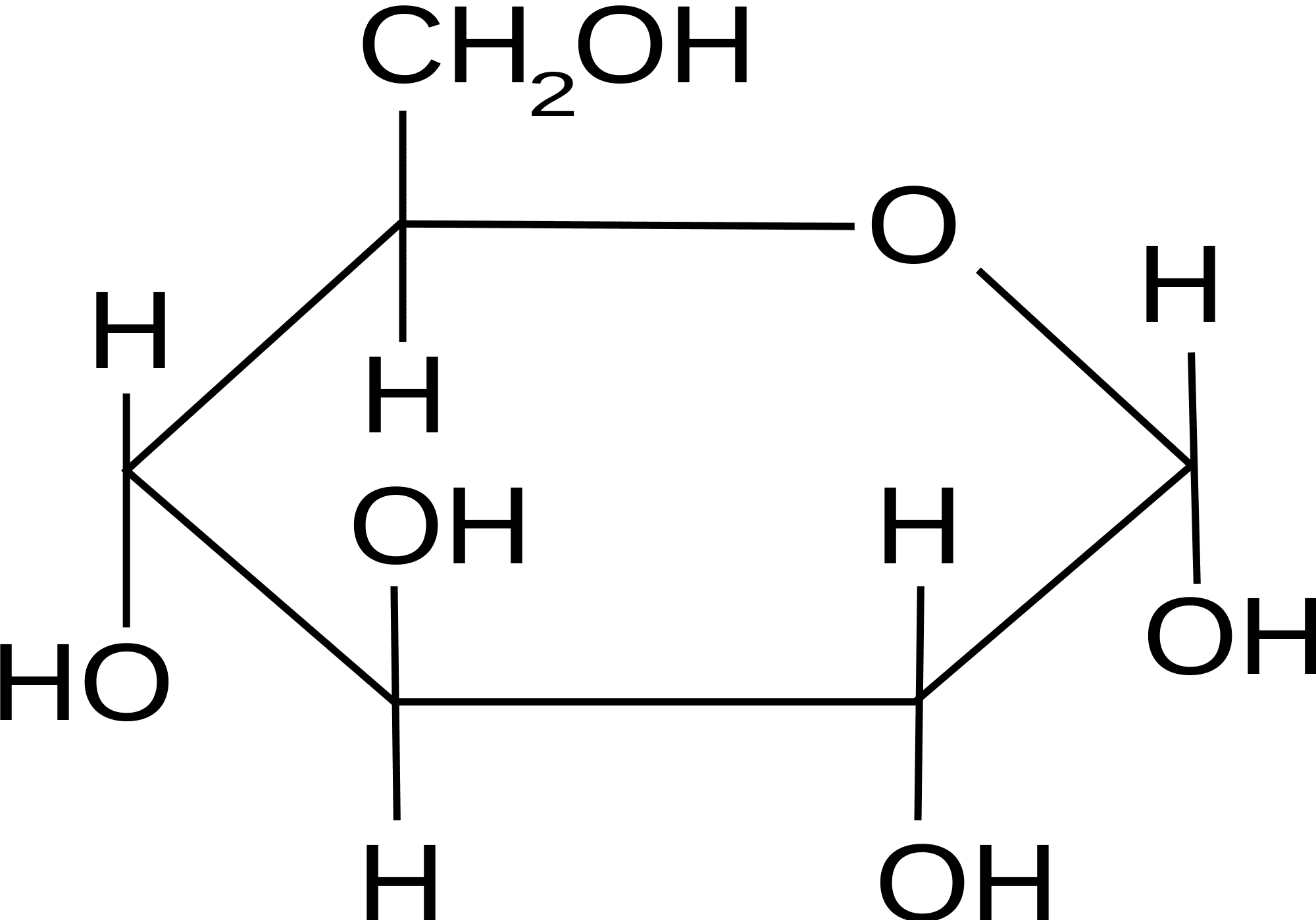
52
New cards
what are the properties of glucose?
* soluble in water
* polar and readily dissolves in water
* transportability
* soluble in water and easily transported in fluids / bloodstreams
* chemical stability
* stable molecule and doesn’t degrade when transported
* energy yield
* glucose is repeatedly oxidized (broken down) to produce ATP in cell respiration
* polar and readily dissolves in water
* transportability
* soluble in water and easily transported in fluids / bloodstreams
* chemical stability
* stable molecule and doesn’t degrade when transported
* energy yield
* glucose is repeatedly oxidized (broken down) to produce ATP in cell respiration
53
New cards
what is polymerization?
condensation and hydrolysis reactions
54
New cards
what are polysaccharides as energy storage compounds?
* they are made of long chains of glucose molecules, combined through condensation reactions
* they have a compact structure due to coiling (amylose) and branching (amylopectin)
* ex. starch in plants and glycogen in animals
* they have a compact structure due to coiling (amylose) and branching (amylopectin)
* ex. starch in plants and glycogen in animals
55
New cards
what is the difference between an alpha-glucose and a beta-glucose?
on the alpha-glucose the H is pointed up on while on the beta-glucose the H is pointed down
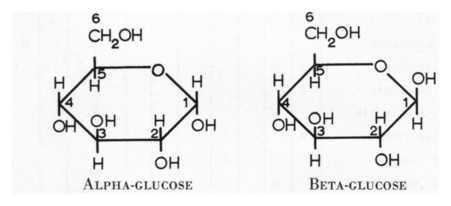
56
New cards
what is starch made of?
* amylose
* amylopectin
* they are large molecules (not very soluble in water)
* amylopectin
* they are large molecules (not very soluble in water)
57
New cards
what, specifically, is amylose?
* long chains of alpha-glucose with glycosidic bonds (carbs) between carbon-1 and carbon-4
* glucose molecules can easily be added to amylose by condensation or removed by hydrolysis
* glucose molecules can easily be added to amylose by condensation or removed by hydrolysis
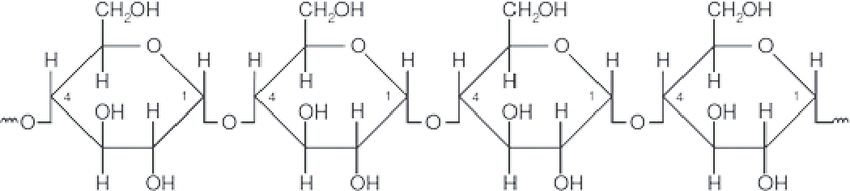
58
New cards
what, specifically, is amylopectin?
* long chains of alpha-glucose with glycosidic bonds between carbon-1 and carbon-4
* at every 20th glucose molecule, an additional glucose molecule bonds at carbon-6 creating a branched structure
* glucose molecules can easily be added to amylopectin by condensation or removed by hydrolysis
* at every 20th glucose molecule, an additional glucose molecule bonds at carbon-6 creating a branched structure
* glucose molecules can easily be added to amylopectin by condensation or removed by hydrolysis
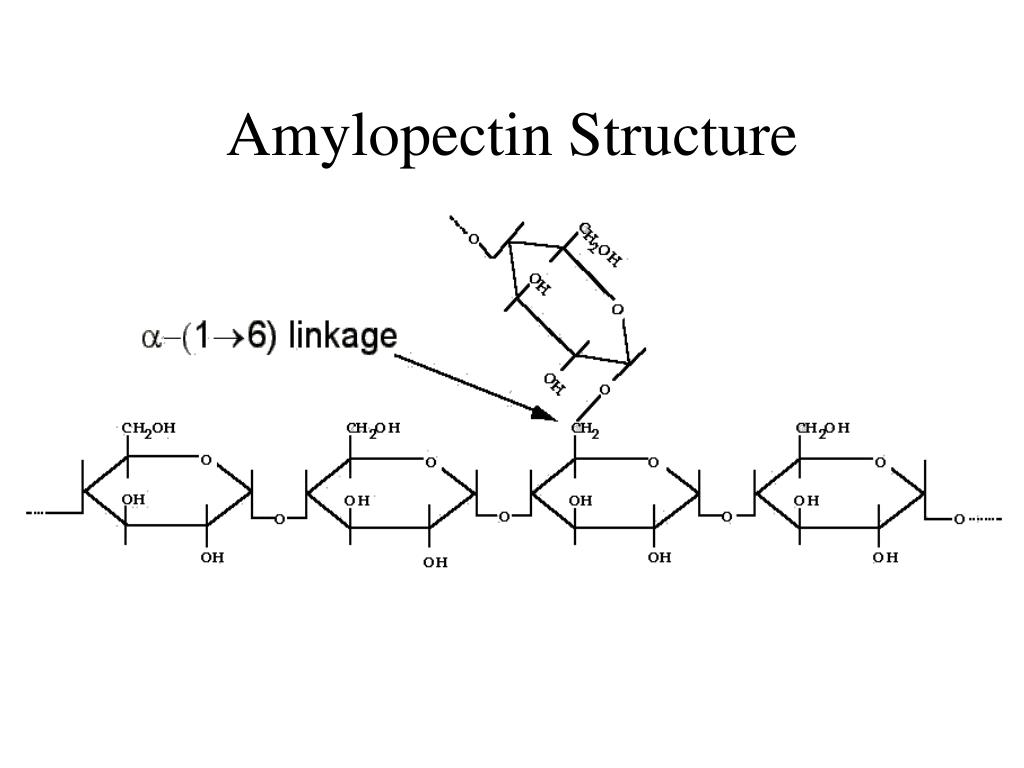
59
New cards
what, specifically, is glycogen?
* short term energy storage in animals (2-5 min worth)
* composed of chains of alpha-glucose with bonds between carbon-1 and carbon-4
* at around every 10th glucose molecule, an additional glucose molecule bonds at carbon-6 creating a highly branched structure
* it has the exact same structure as amylopectin except it is more branched
* composed of chains of alpha-glucose with bonds between carbon-1 and carbon-4
* at around every 10th glucose molecule, an additional glucose molecule bonds at carbon-6 creating a highly branched structure
* it has the exact same structure as amylopectin except it is more branched
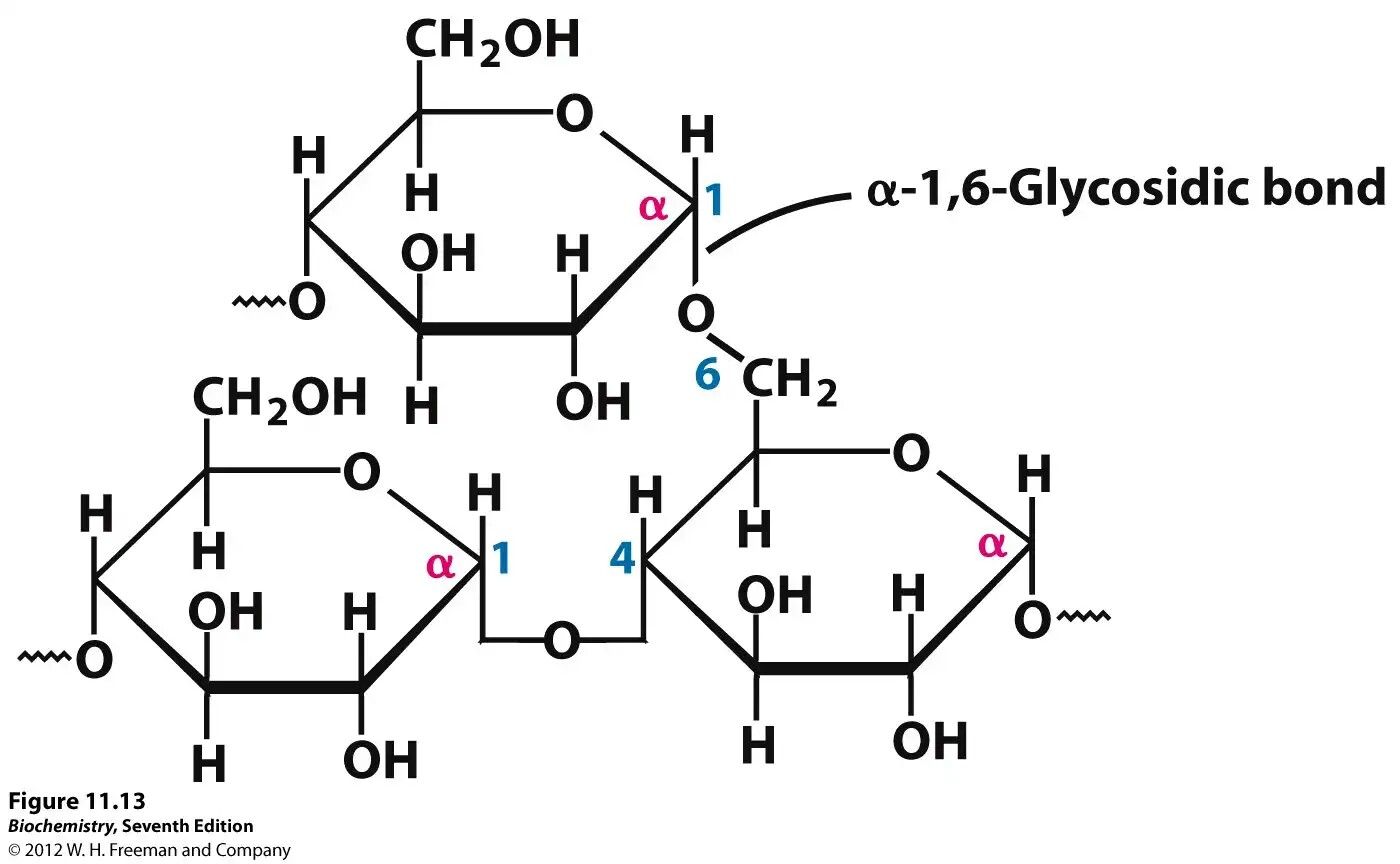
60
New cards
what is cellulose?
* makes up the structure of plant cell walls
* is commonly referred to as dietary fiber
* it is made up of beta-glucose molecules
* is commonly referred to as dietary fiber
* it is made up of beta-glucose molecules
61
New cards
what does cellulose look like?
* it has an unbranched structure
* every other molecule is flipped resulting in straight chains of cellulose
* every other molecule is flipped resulting in straight chains of cellulose
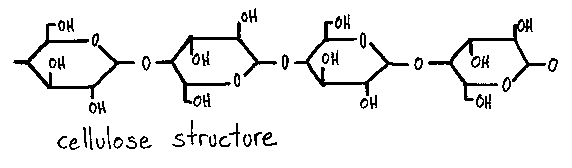
62
New cards
what are microfibrils?
* cellulose chains bonded together through hydrogen bonds
* they have high tensile strength that allows them to maintain the structure of the plant cell walls
* they have high tensile strength that allows them to maintain the structure of the plant cell walls
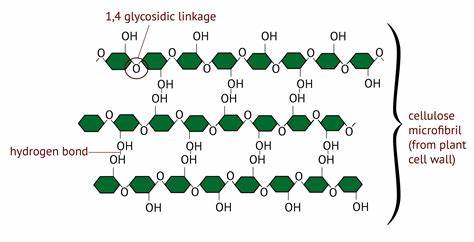
63
New cards
what are glycoproteins?
* carbohydrates bonded to a protein
* they are found in the phospholipid bilayer of the cell membrane
* it has specific structure and functions
* they are found in the phospholipid bilayer of the cell membrane
* it has specific structure and functions
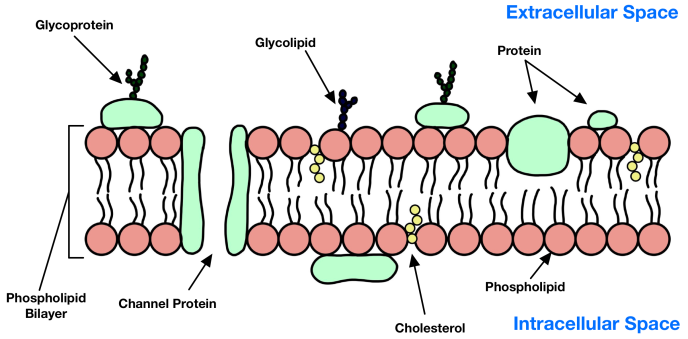
64
New cards
what are the roles of the glycoproteins?
* cell to cell adhesion
* they interact with glycoproteins on neighboring cells, allowing the formation of tissue
* cell to cell communication
* neurotransmitters bind to glycoproteins allowing communication between cells
* immune response
* they act as ID tags to help the immune system to distinguish between self and non-self cells
* they interact with glycoproteins on neighboring cells, allowing the formation of tissue
* cell to cell communication
* neurotransmitters bind to glycoproteins allowing communication between cells
* immune response
* they act as ID tags to help the immune system to distinguish between self and non-self cells
65
New cards
what is the ABO Blood Groups?
* glycoproteins act as antigens (molecules that stimulate an immune response and the production of antibodies)
* red blood cells can have anitgen A, antigen B, or both
* red blood cells can have anitgen A, antigen B, or both
66
New cards
which blood has which antigens?
* blood type A
* has anti-B antibodies
* has A antigen
* blood type B
* has anti-A antibodies
* has B antigen
* blood type AB
* has no antibodies
* has both A and B antigens
* blood type O
* has both anti-A and anti-B antibodies
* has no antigens
* has anti-B antibodies
* has A antigen
* blood type B
* has anti-A antibodies
* has B antigen
* blood type AB
* has no antibodies
* has both A and B antigens
* blood type O
* has both anti-A and anti-B antibodies
* has no antigens
67
New cards
WHAT IS THE TESTING FORMAT????
* MULTIPLE CHOICE
* DATA ANALYSIS
* A FEW SHORT ANSWER
* ONE LONG ANSWER
* DATA ANALYSIS
* A FEW SHORT ANSWER
* ONE LONG ANSWER