3B enthalpy change
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
What is enthalpy
The measure of the heat energy in a chemical system
What is enthalpy change
The difference in enthalpy between reactants and products in chemical reactions
How to know if enthalpy reaction is exothermic
If reactants is higher than products, energy is released into surroundings as heat. This means it is exothermic
ΔH for exothermic is negative
Making bonds is exothermic
Temperature change is positive
How to know if an enthalpy reaction is endothermic
If the reactants are lower than the products, energy will be taken in from surroundings
ΔH For endothermic is positive
Breaking bonds is endothermic
Temperature change is negative
energy profile diagrams for both endothermic and exothermic enthalpy
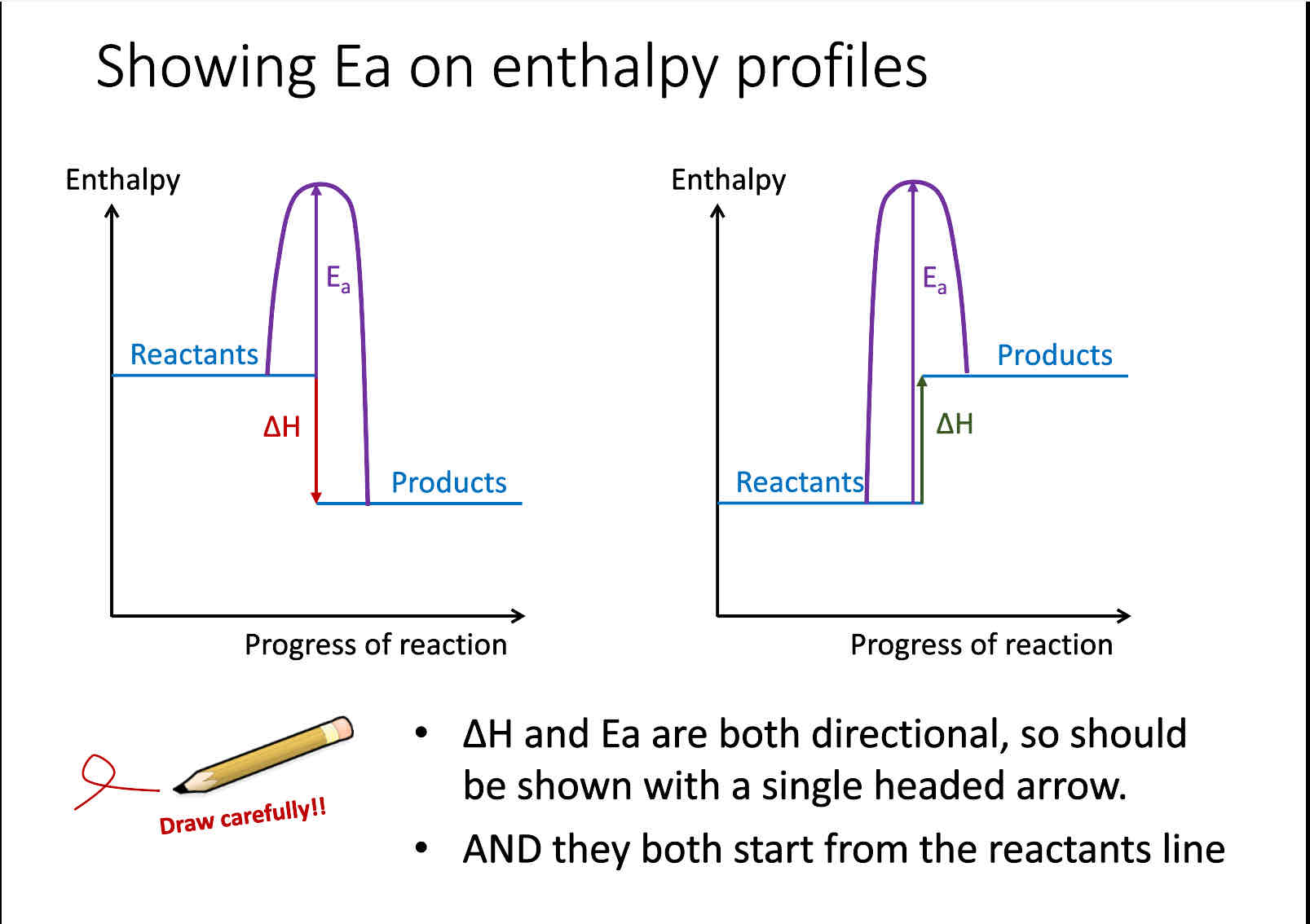
What is activation energy
Minimum energy required for a reaction to take place, it is required to break bonds in reactants
what is the formula for energy in calorimetry calculations q= mcΔT
Q = energy exchanged around surroundings (in joules)
M = mass of substance heated (in grams)
C = specific heat capacity (in J g -1 C -1 or with K) usually 4.18
T = temperature change (can be in either Celsius or kelvin)
ΔH What is enthalpy change formula and what is its formula
- q in kj / mol
Units = Kj mol -1
What are the 2 methods for measuring heat transfer
For aqueous solution
Methods
•carry out reaction, using known amount of reactant , use unknown volume of water to absorb/provide energy, measure temperature change
measurements - mass /volume of starting material
volume of water / solution
temperature
procedure
•Heat loss to surroundings
•energy to heat up equipment
Method - for combustion
•burn a known amount of reactant
•use known volume of water to absorb energy
•measure temperature change
measurements = measure material burned, volume of water, temperature change
procedure = heat loss to surroundings, energy to heat up equipment, incomplete combustion
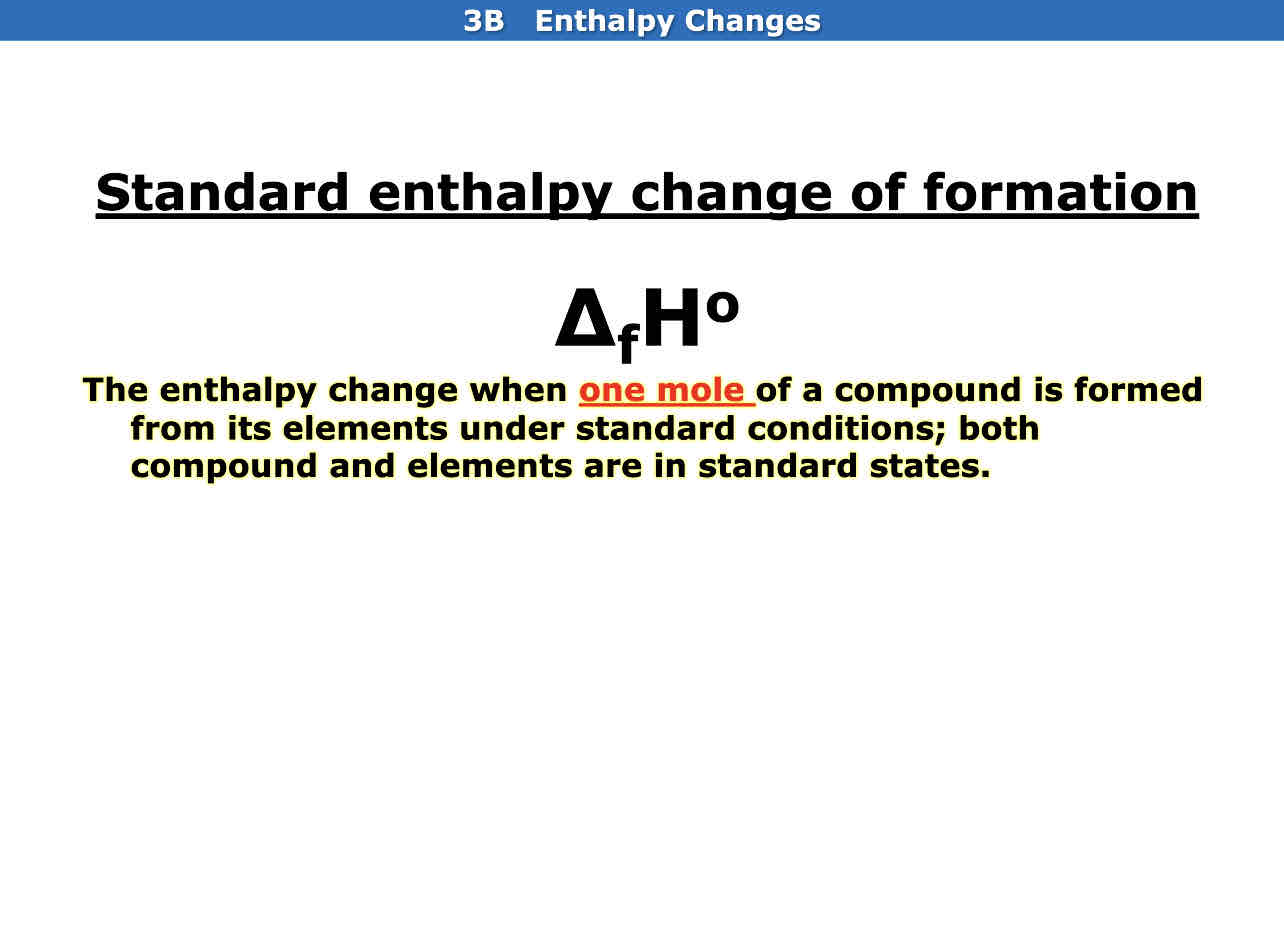
What is standard enthalpy change of formation
The enthalpy change when one mole of a compound is formed from its elements under standard conditions; both compounds and elements are in standard states
What is standard enthalpy change of combustion
The enthalpy change when one mole of an element or compound reacts completely with oxygen Under standard conditions

What is standard enthalpy change of neutralisation
The enthalpy change when 1 mole of water is formed from neutralisation of hydrogen ions by hydroxide ions under standard conditions
What is standard enthalpy change of atomisation
The enthalpy change when one mole of gaseous atoms is formed from the element in its standard state
What is average bond enthalpy
breaking of one mole of bonds
Into gaseous molecules
What does homolytically mean
Equally
In bond enthalpy what sign is endothermic reaction and why
+ve
Bond breaking requires an input of energy
In bond enthalpy what sign is exothermic reaction and why
-ve
Forming bonds releases energy
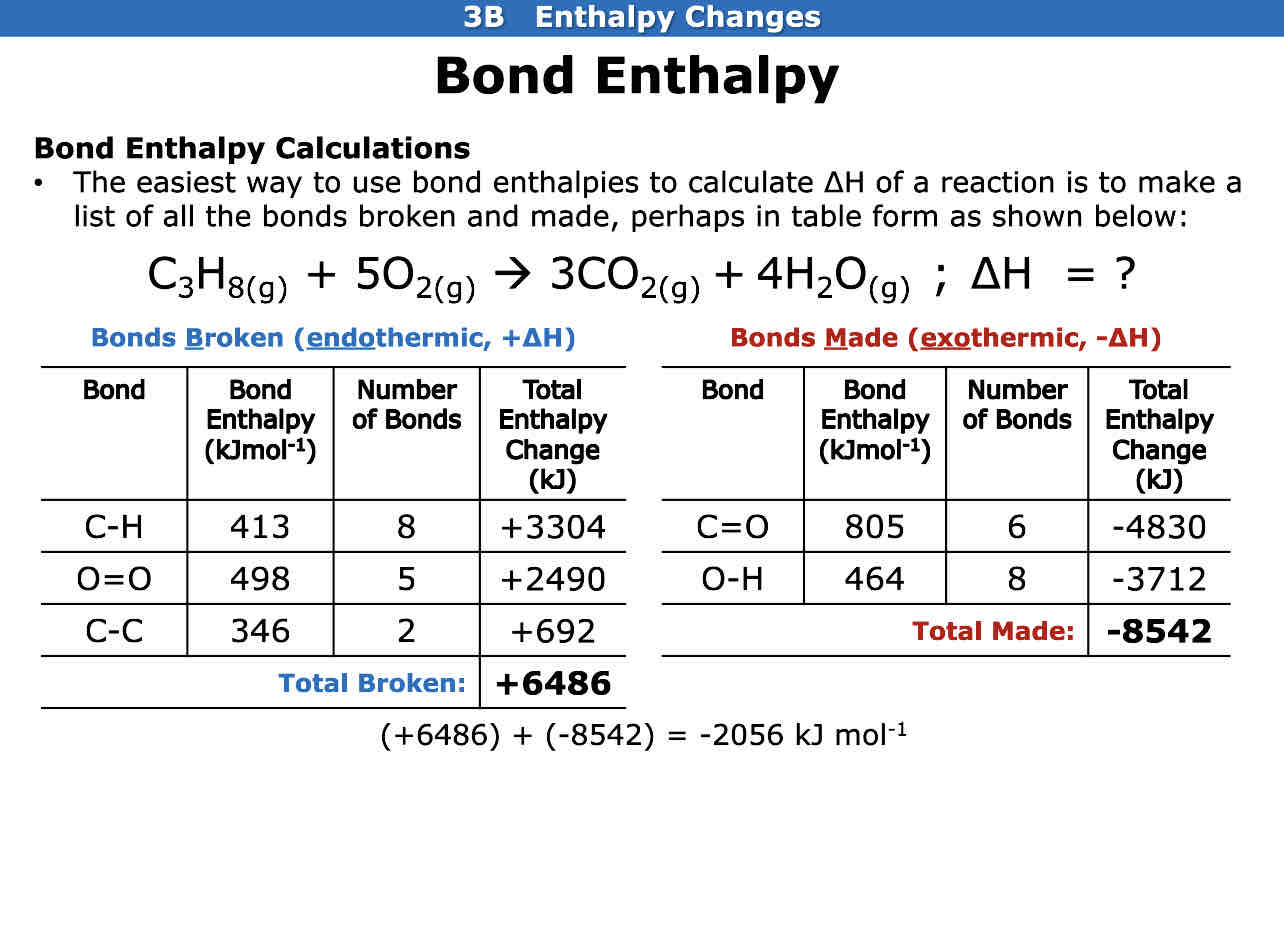
How do we do bond enthalpy calculations
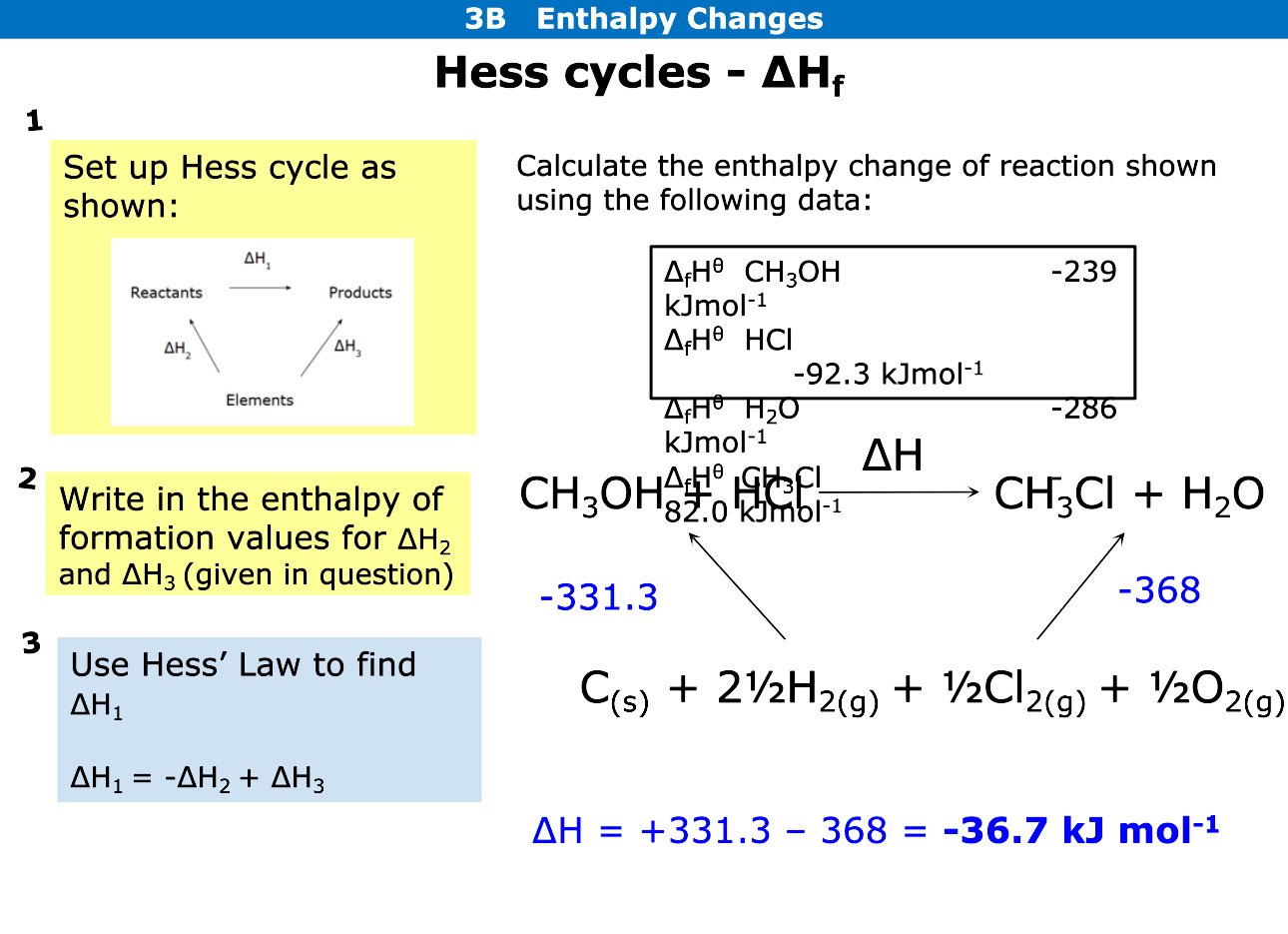
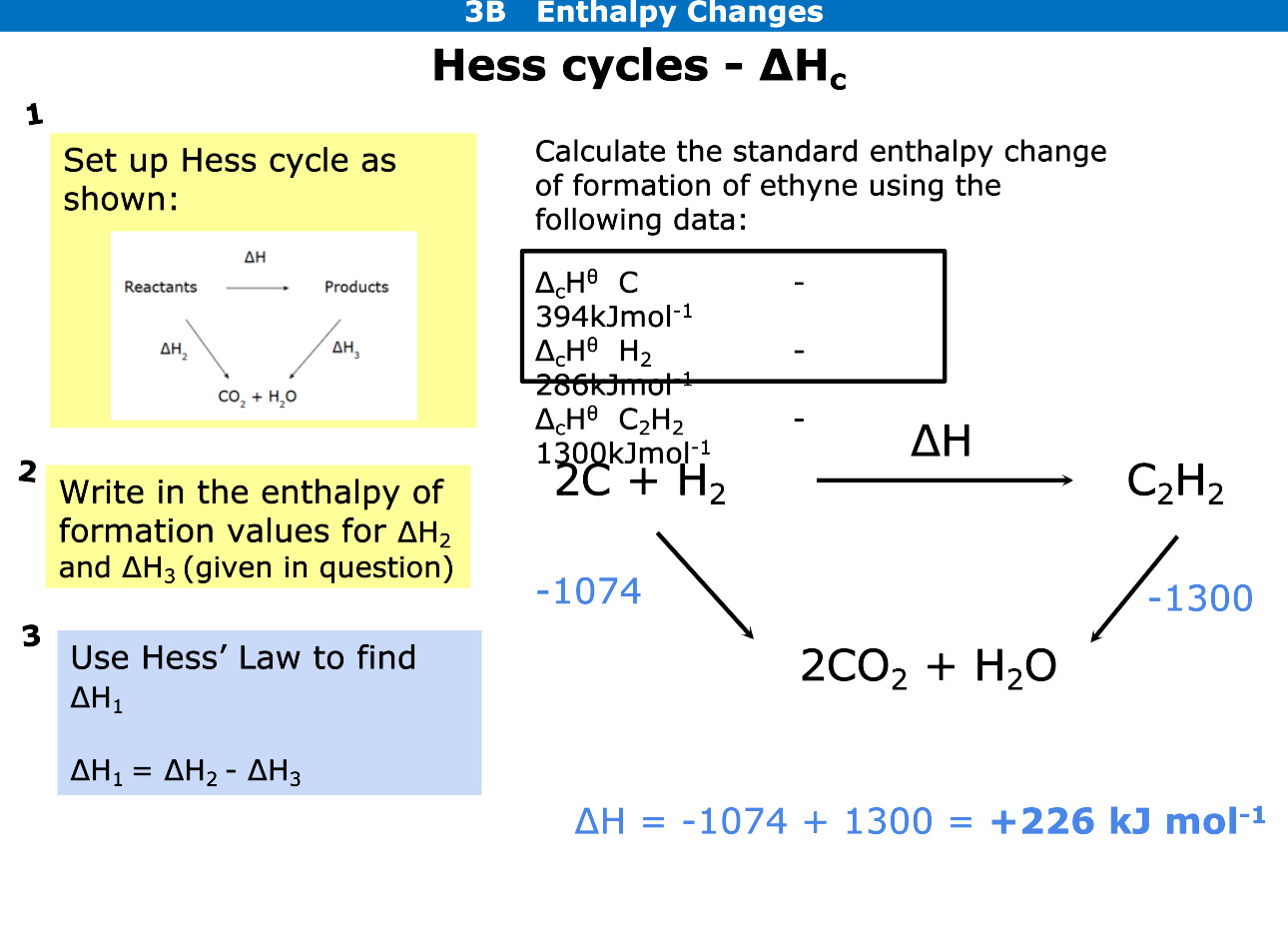
Using Hess cycles how do you calculate enthalpy change of formation
For formation arrows are pointed downwards
Write the elements beneath reaction
Add up elements on left and see if arrow going with reaction or against, if against change sign
Add up elements on right and work out formation
In Hess cycles how do you calculate enthalpy of combustion
For combustion arrows are pointed downwards
Beneath write CO2 and H2O
Add up elements on left and see if arrow going with reaction or against, if against change sign
Add up elements on right and work out formation
What is the formula for enthalpy change of neutralisation
How do we work out enthalpy change of neutralisation
Formula =
Delta H = -m x c x change in temperature
Add the volumes as they are equal to mass and divide by 1000
Use 4.18
Use temperature
Gives you value which is enthalpy of reaction
Work out moles of water using moles= conc x volume
Moles of acid is = moles of water
Enthalpy value/moles of water = enthalpy of neutralisation

How do you calculate maximum temperature reached
Calculate moles by doing concentration x volume
Find energy released by doing delta H (given) x moles
change in temp = Q (just worked out) / mass x 4.18 (C)
Gives you temp value
Maximum temp = new temp value + temp value in question
How to work out enthalpy of combustion or enthalpy of formation
Enthalpy of formation/combustion = reactants - products