AP Chem Test 2B
1/18
Earn XP
Description and Tags
Topics 2.5 to 2.7
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
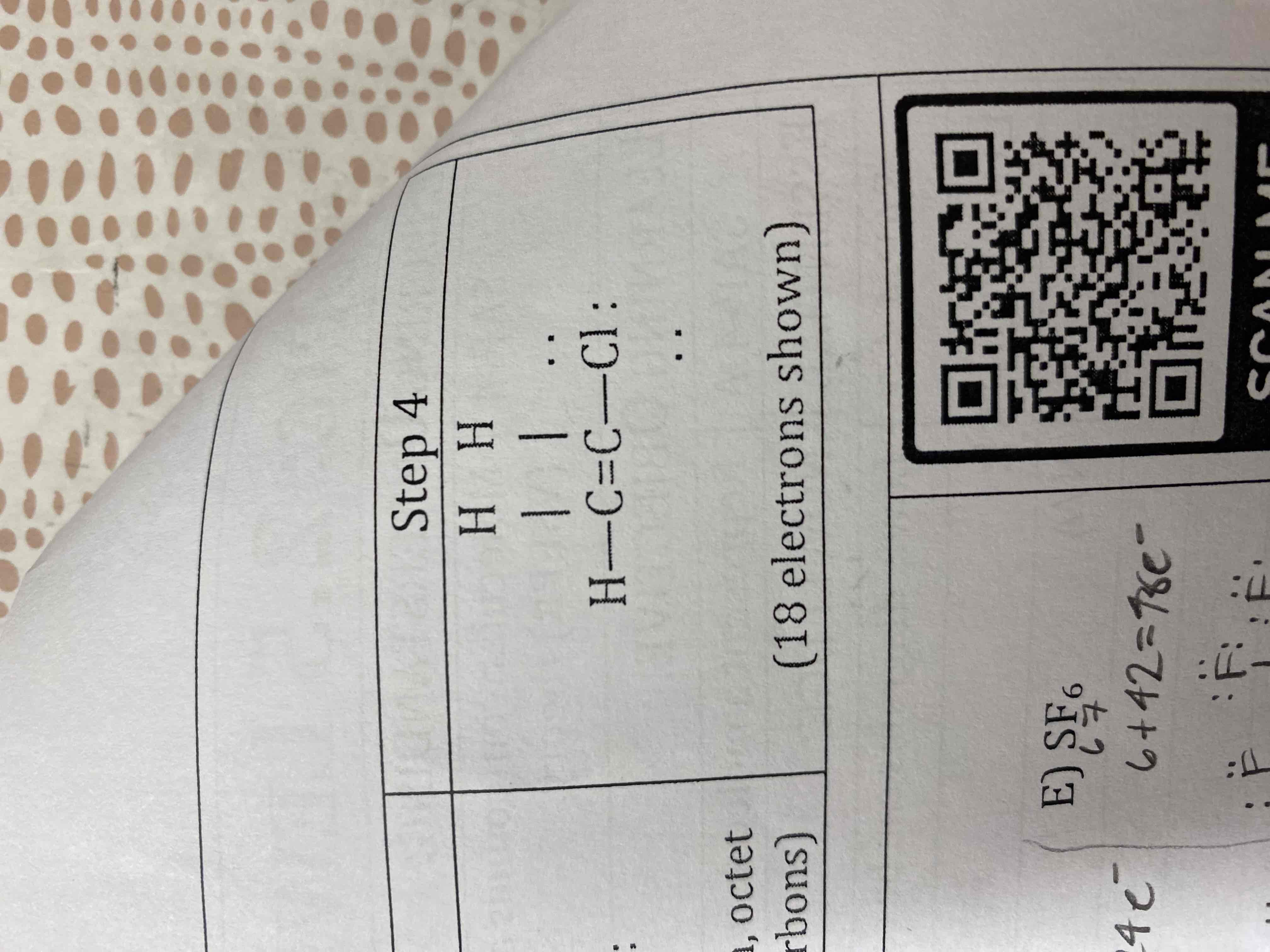
Draw a Lewis Diagram for C2H3Cl
Draw a Lewis Diagram for the following:
A) C2H2
B) OH-
C) CF2S
D) BCl3
E) SF6

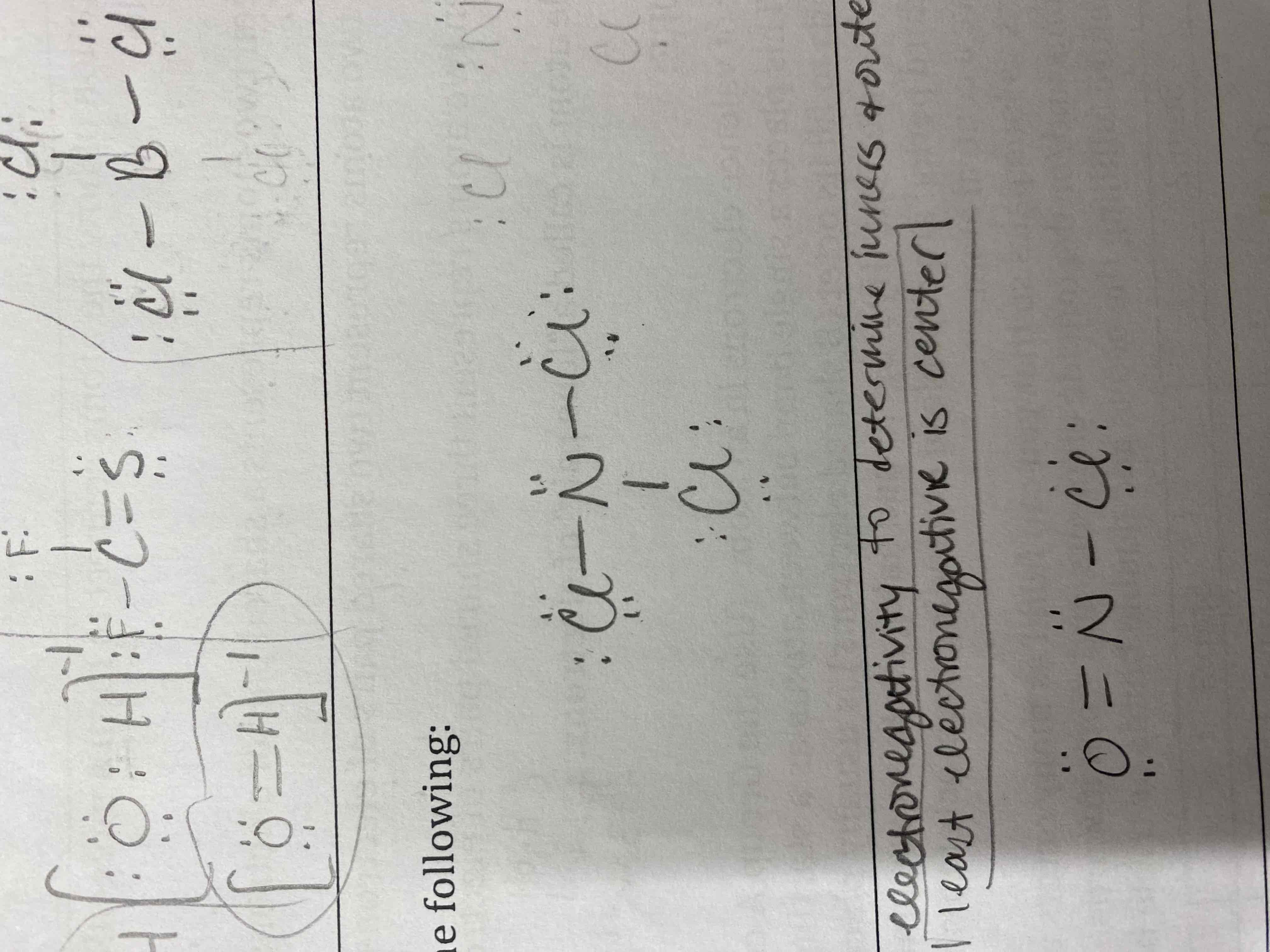
Draw a Lewis Diagram for the following:
1) NCl3
2) NOCl

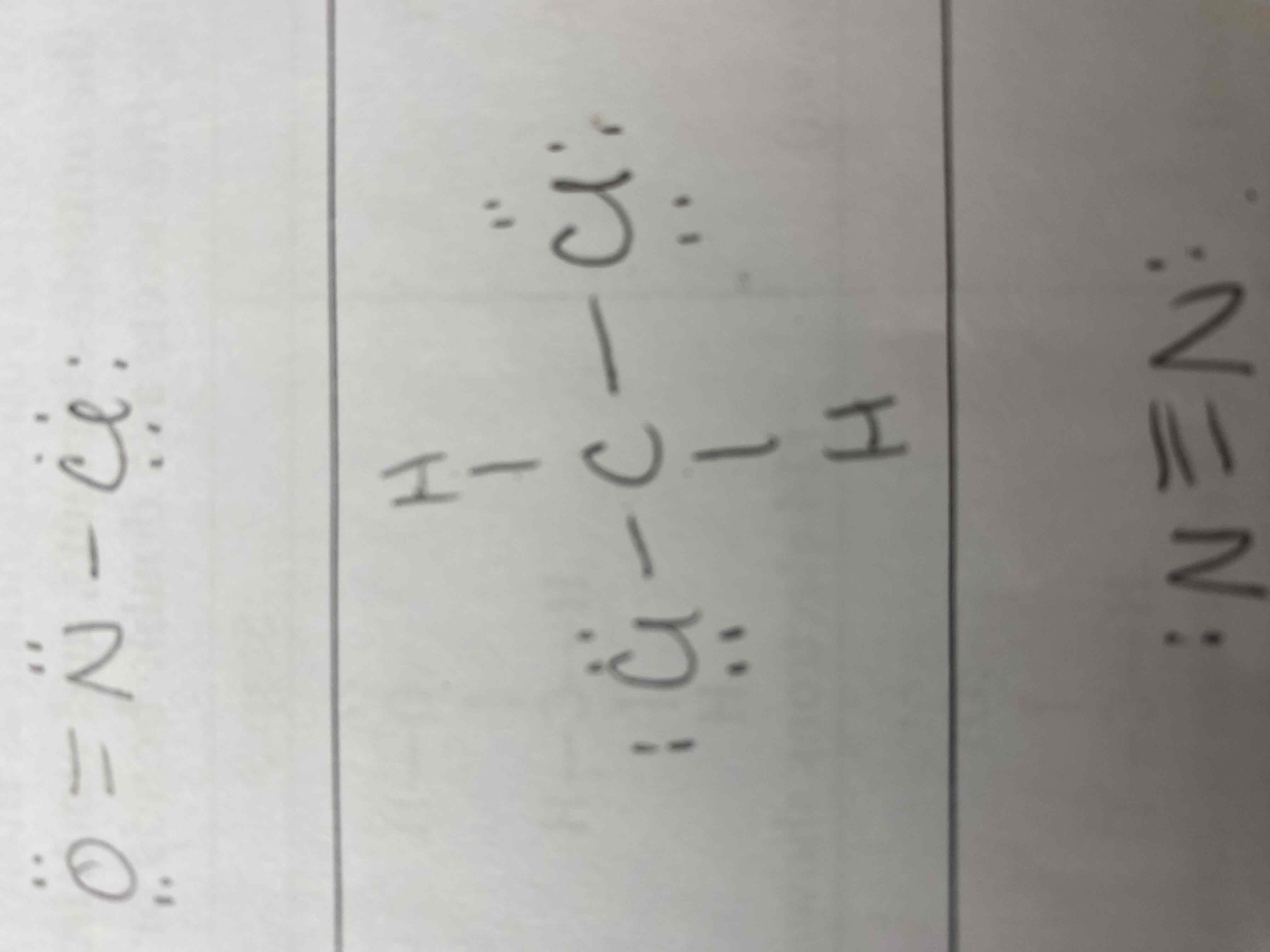
3) CH2Cl2
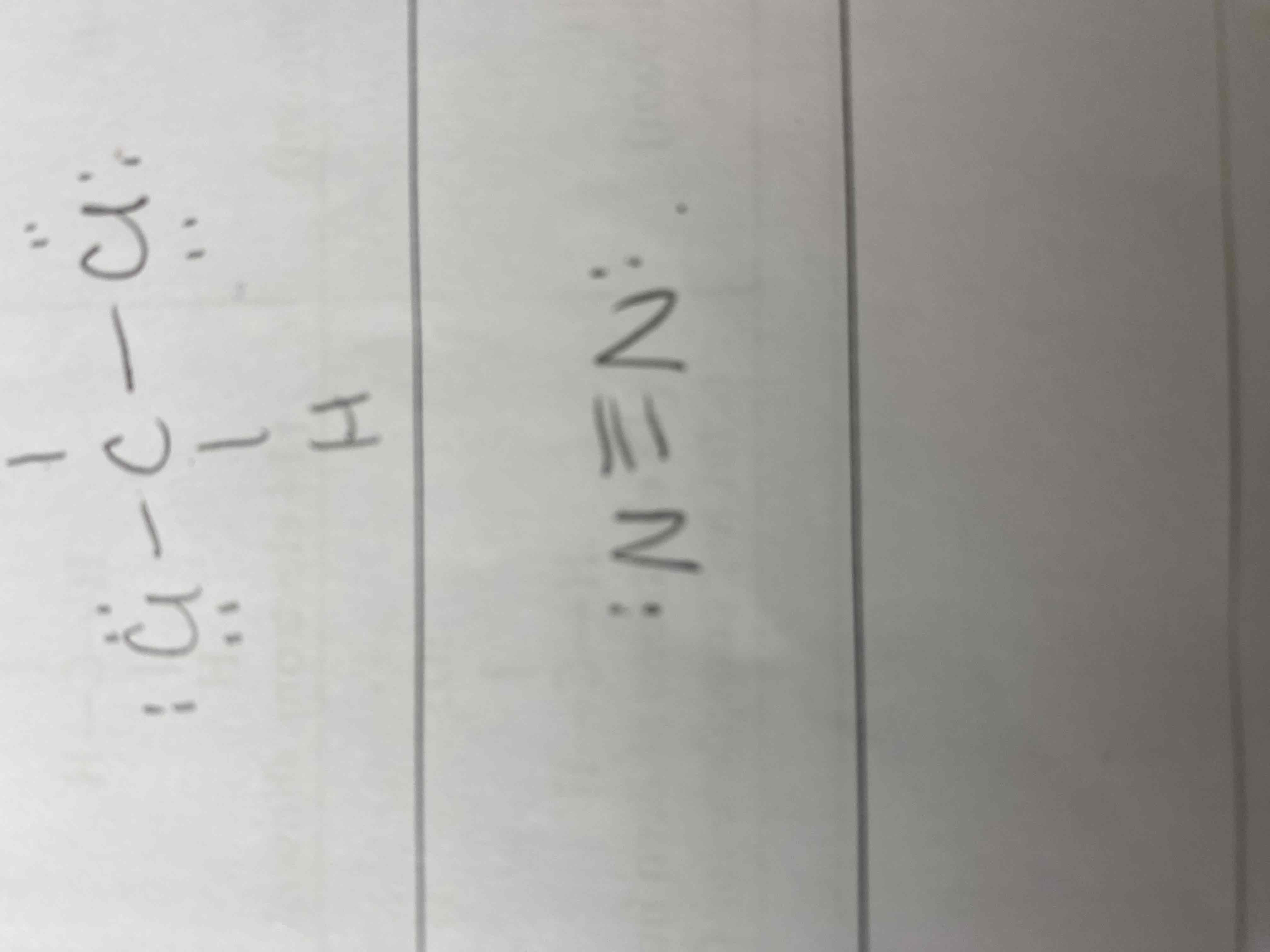
4) N2
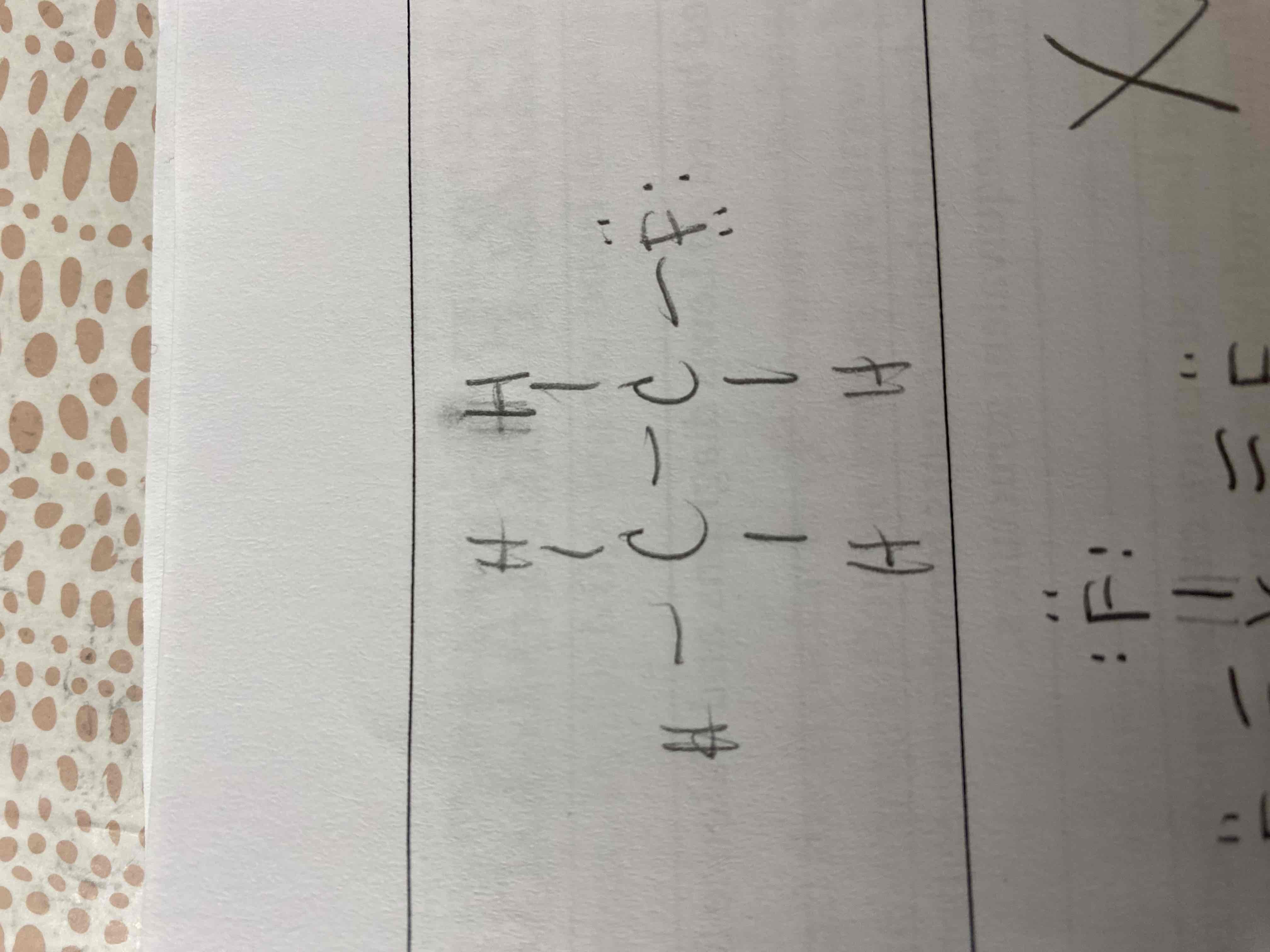
5) C2H5F
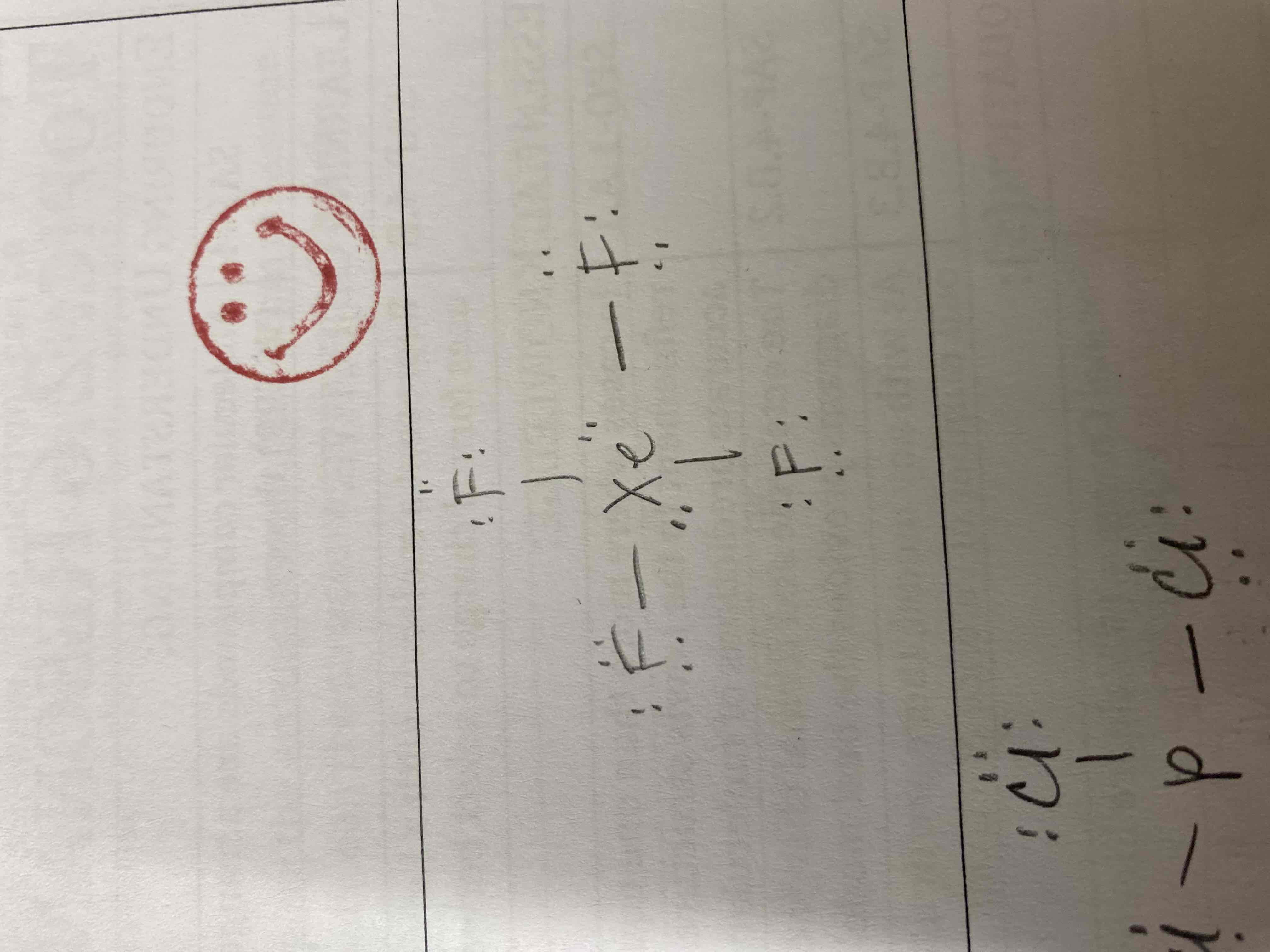
6) XeF4
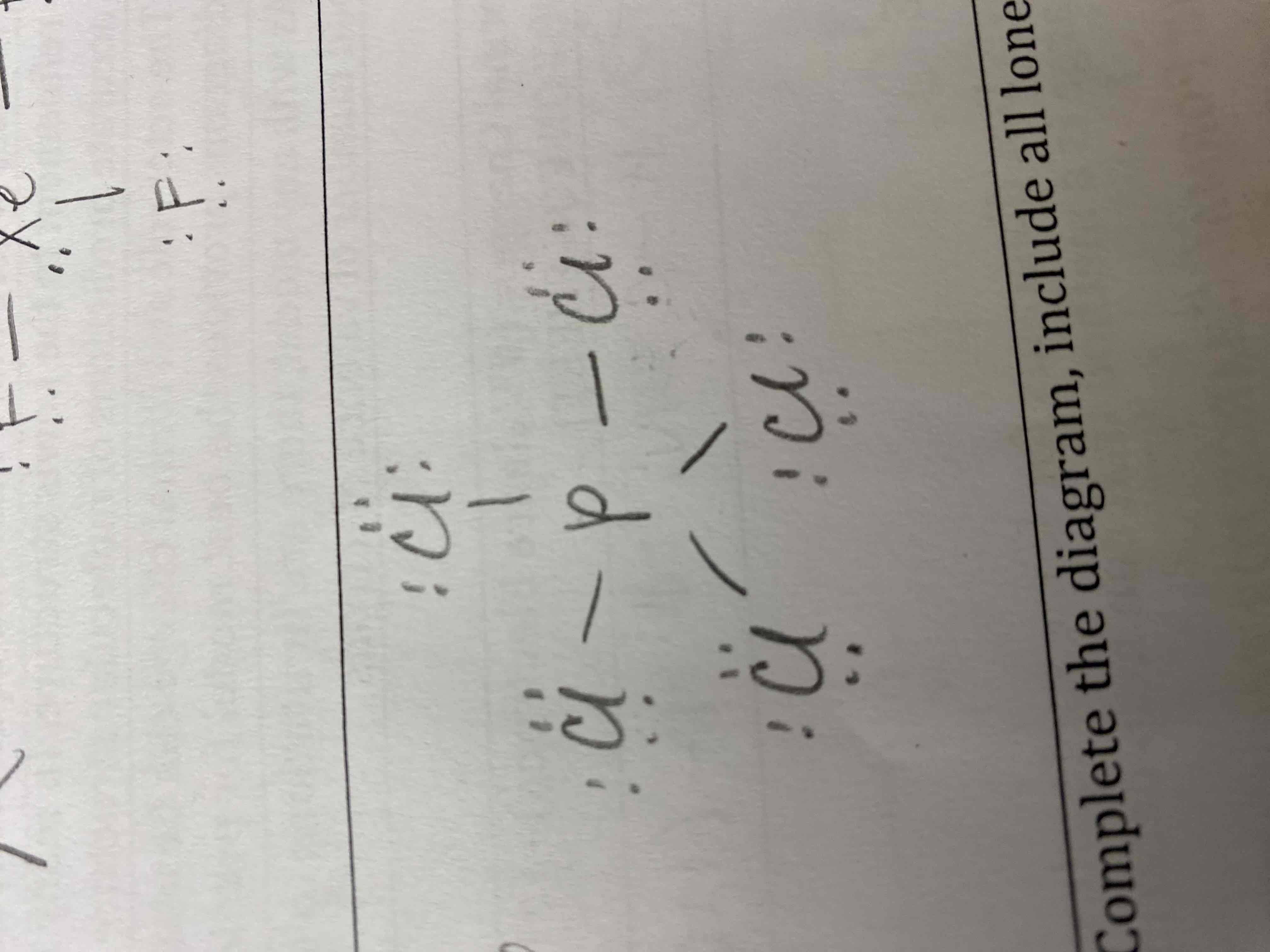
7) PCl5

8) Butyric Acid, C4H8O2, is partially shown below. Complete the diagram, include all lone pairs.
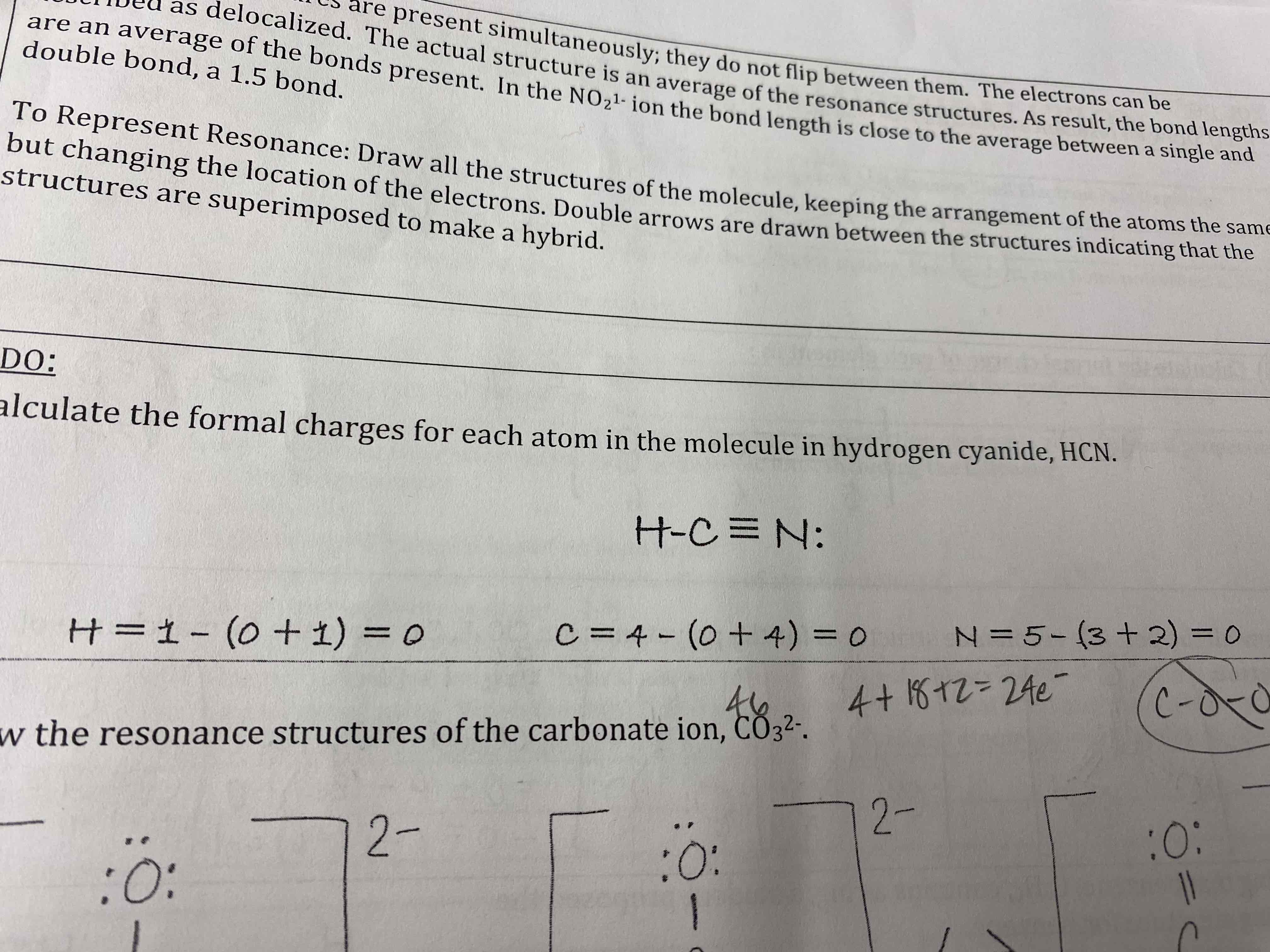
Calculate the formal charges for each atom in the molecule in hydrogen cyanide, HCN.
Draw the resonance structures for the carbonate ion, CO3²-
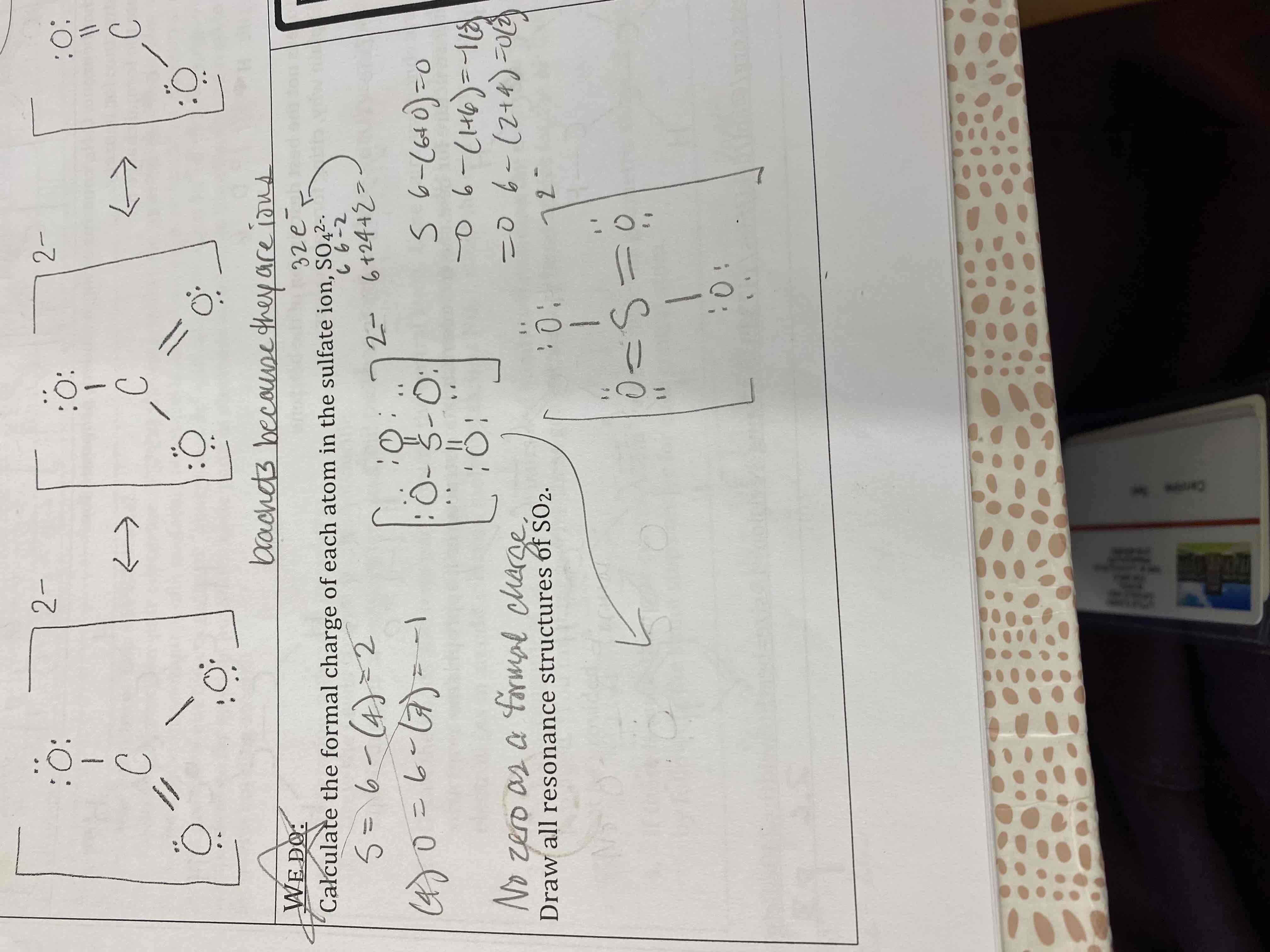
Calculate the formal charge of each atom in the sulfate ion, SO4²-.
Draw all resonance structures of SO2.
Draw all resonance structures for Ozone, O3.
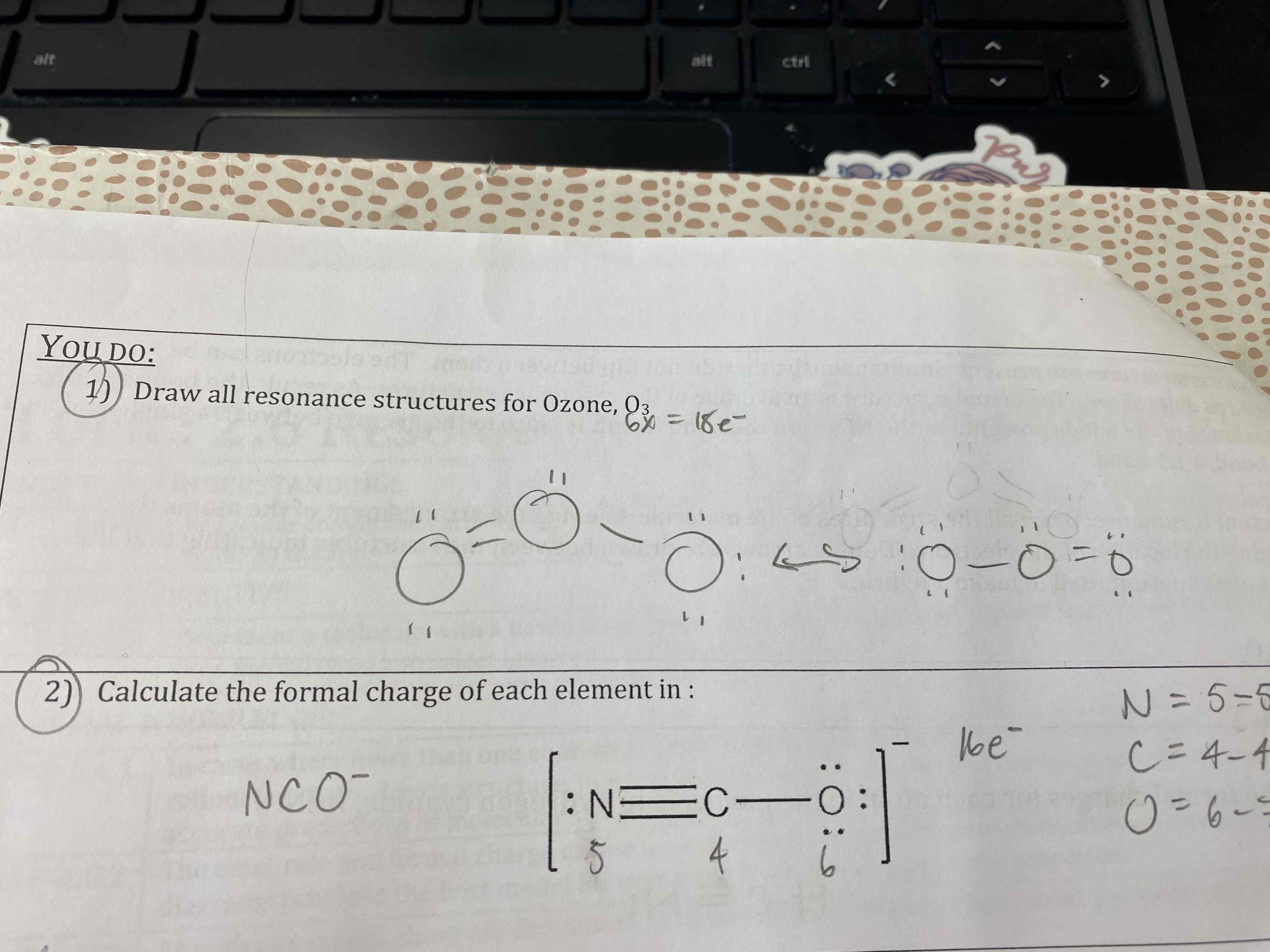
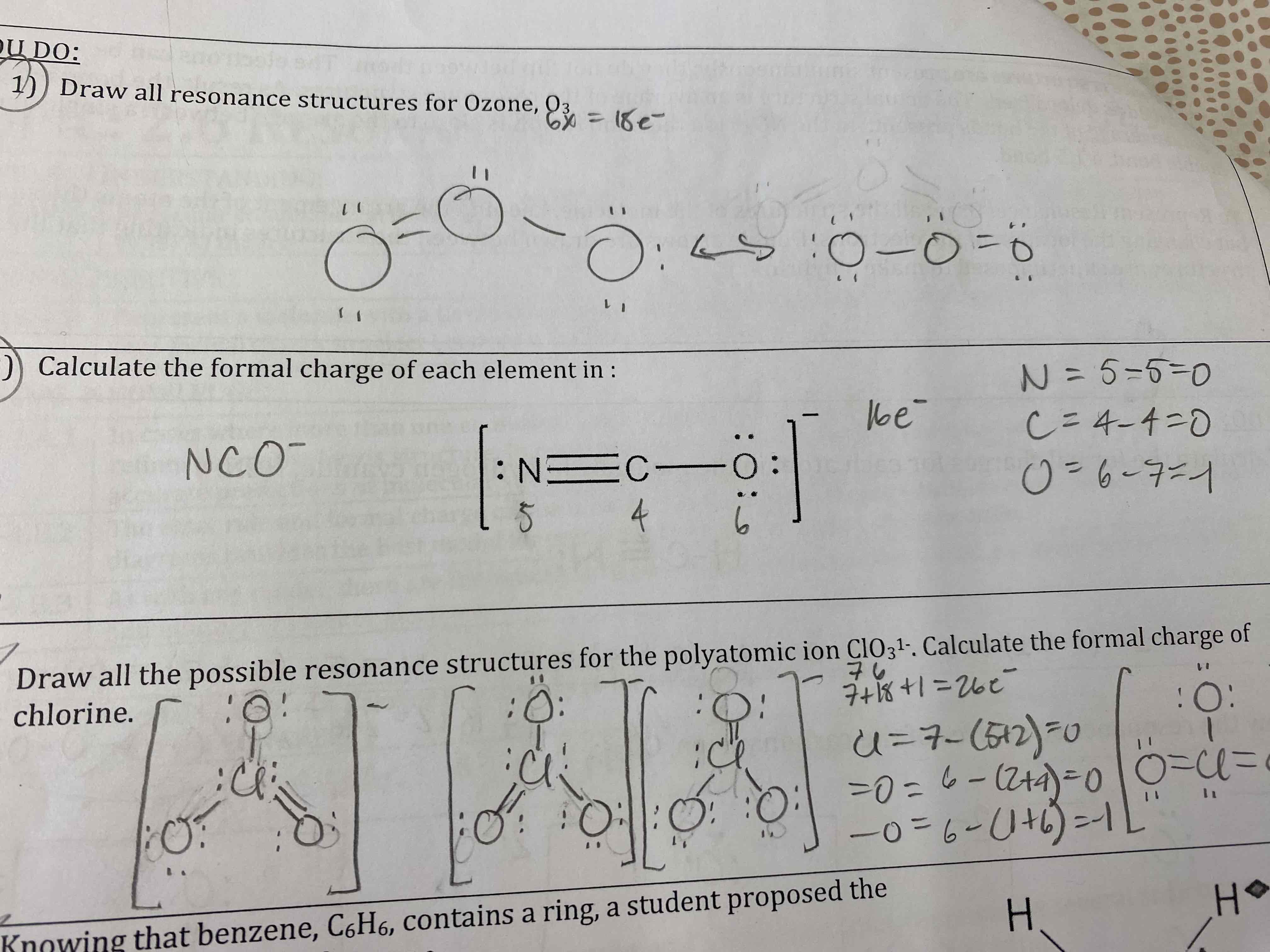
Calculate the formal charge of each element in:
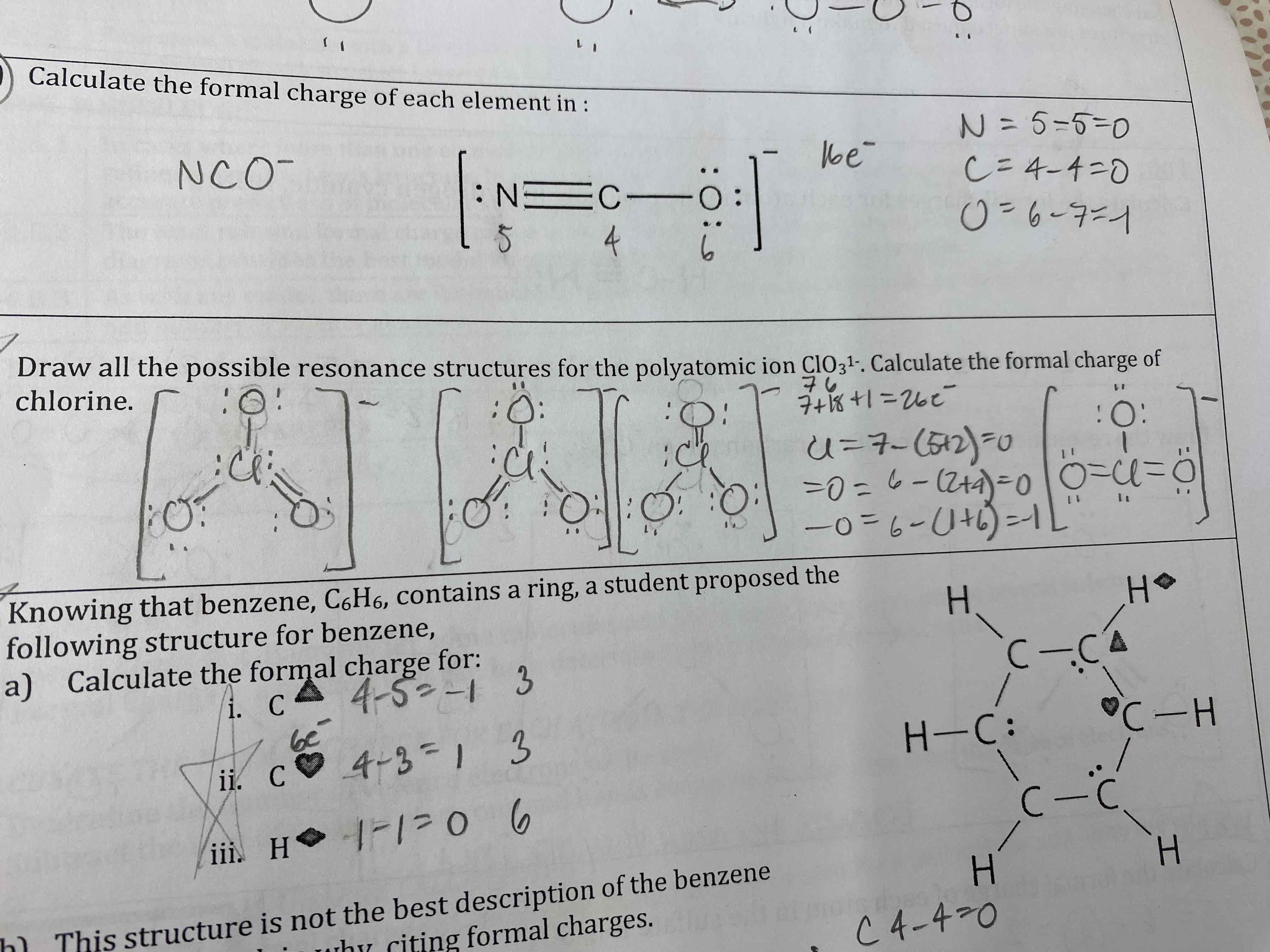
Draw all possible resonance structures for the polyatomic ion ClO3^1-. Calculate the formal charge of chlorine.
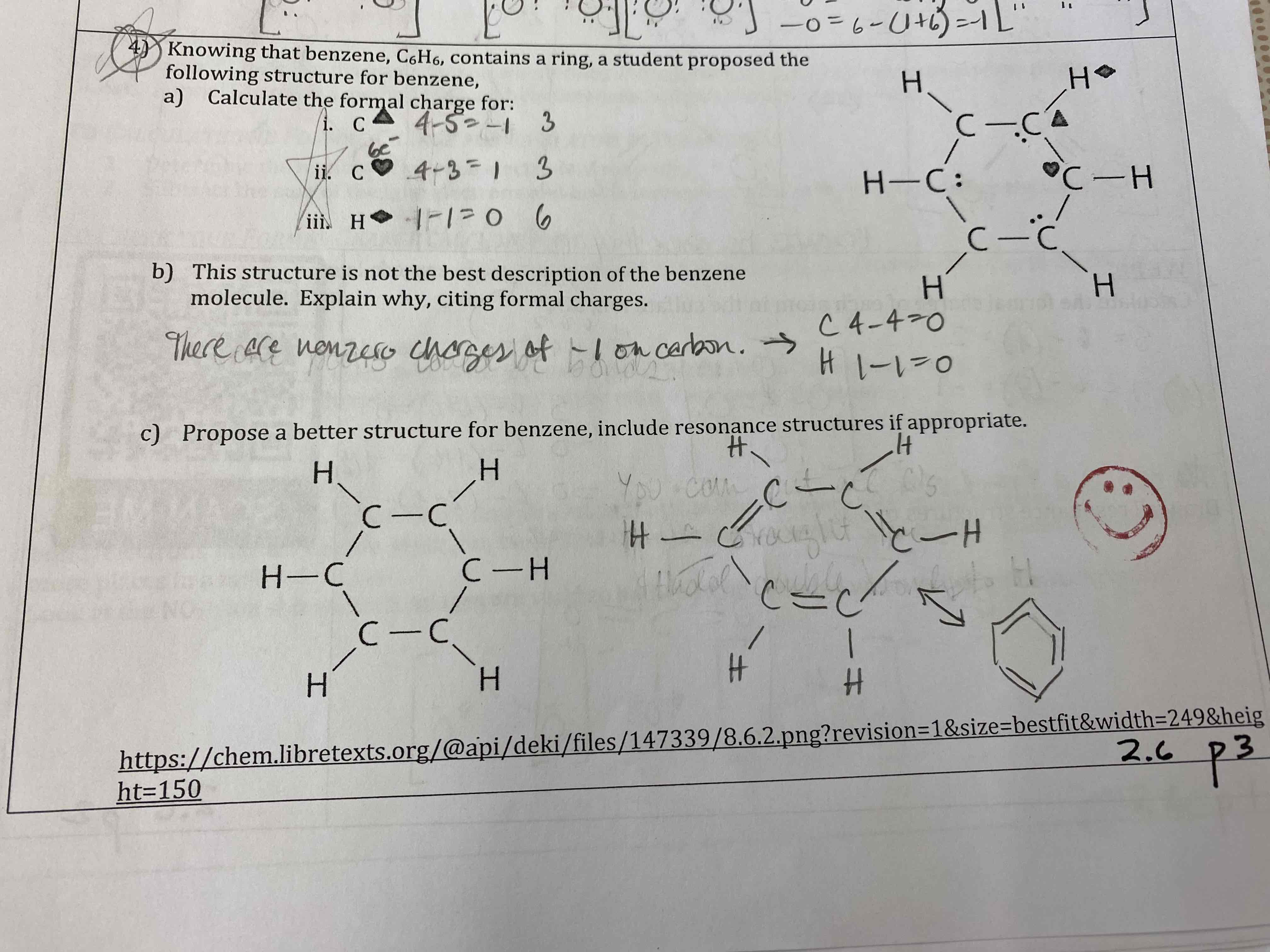
Knowing that benzene, C6H6, contains a ring, a student proposed the following structure for benzene.
a) Calculate the formal charge for:
b) This structure is not the best description of the benzene molecule. Explain why, citing formal charges.
c) Propose a better structure for benzene, include resonance structures if appropriate.

Determine the molecular geometry and predict the bond angles of the following:
A) ICl2-
B) SOF4
Draw a Lewis structure forbyhe following chemical species. Indicate the hybridization on the central atom and the total number of sigma and pi bonds in the molecule.
A) CN-
B) H2CO
Is ammonia, NH3, polar or nonpolar? Explain using molecular shape, polar bonds and indicate if the dipoles cancel using arrows.
Consider the Lewis structures for ethane and ethene below.
The carbon-carbon bond in which molecule has the highest bond order?
The carbon-carbon bond in which molecule has the lowest bond energy?
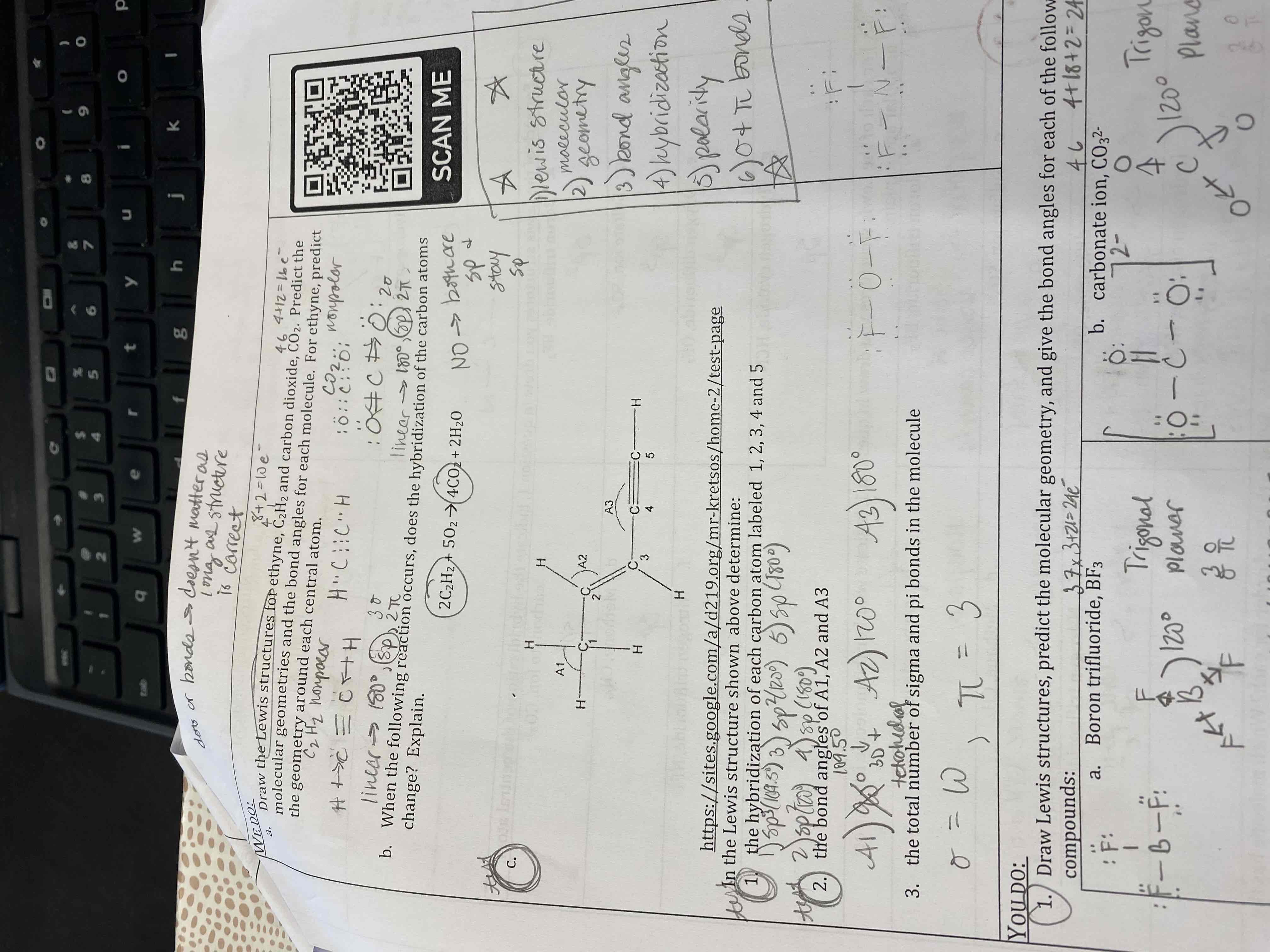
A) Draw the Lewis structures for ethane, C2H2 and carbon dioxide, CO2. Predict the molecular geometries and the bond angles for each molecule. For ethyne, predict the geometry around each central atom.
B) when the following reaction occurs, does the hybridization of the carbon atoms change? Explain.
C) In the Lewis structure shown above determine:
1. the hybridization of each carbon atom labeled 1, 2, 3, 4 and 5
2. the bond angles of A1, A2 and A3
3. the total number of sigma and pi bonds in the molecule.
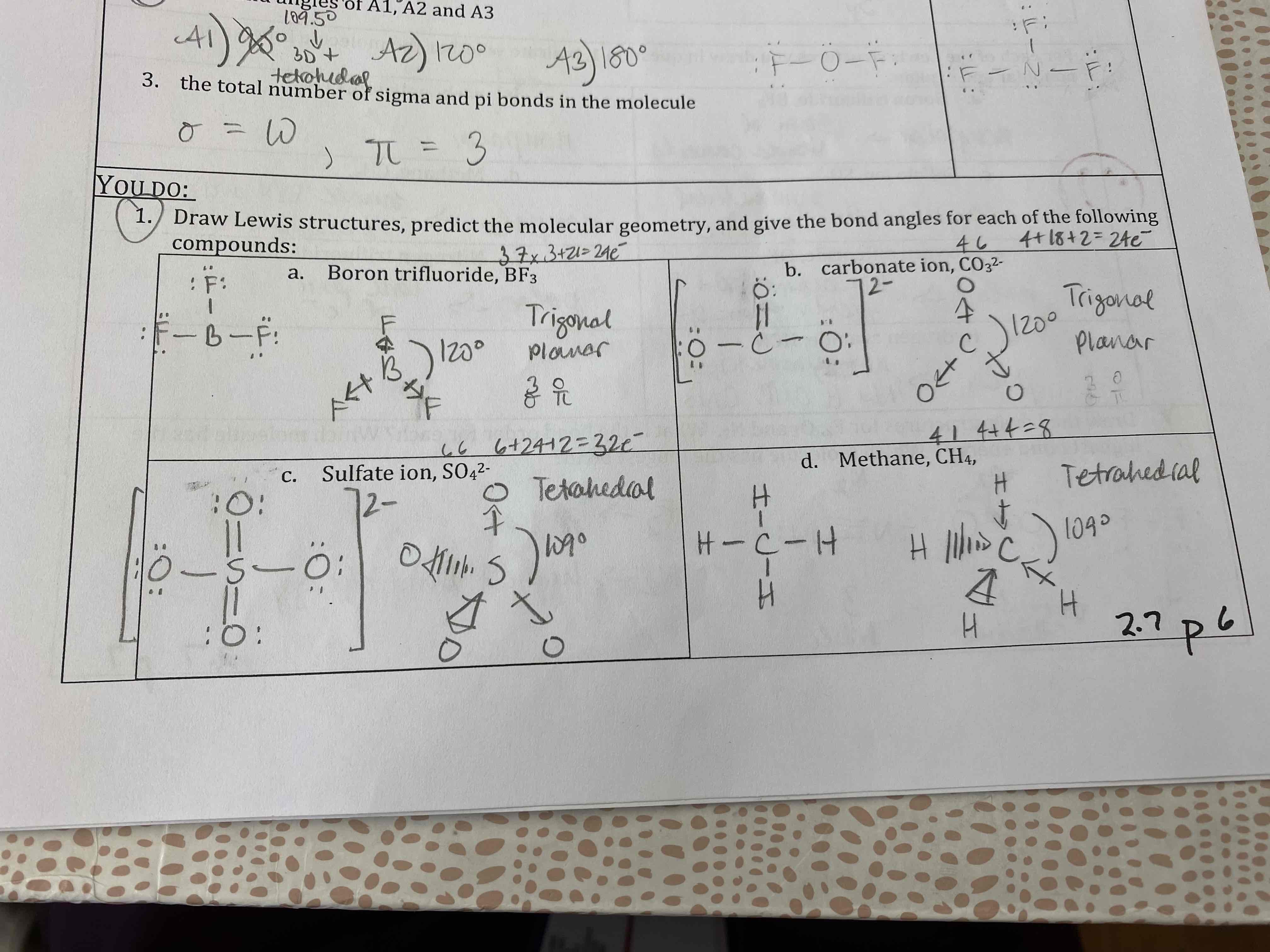
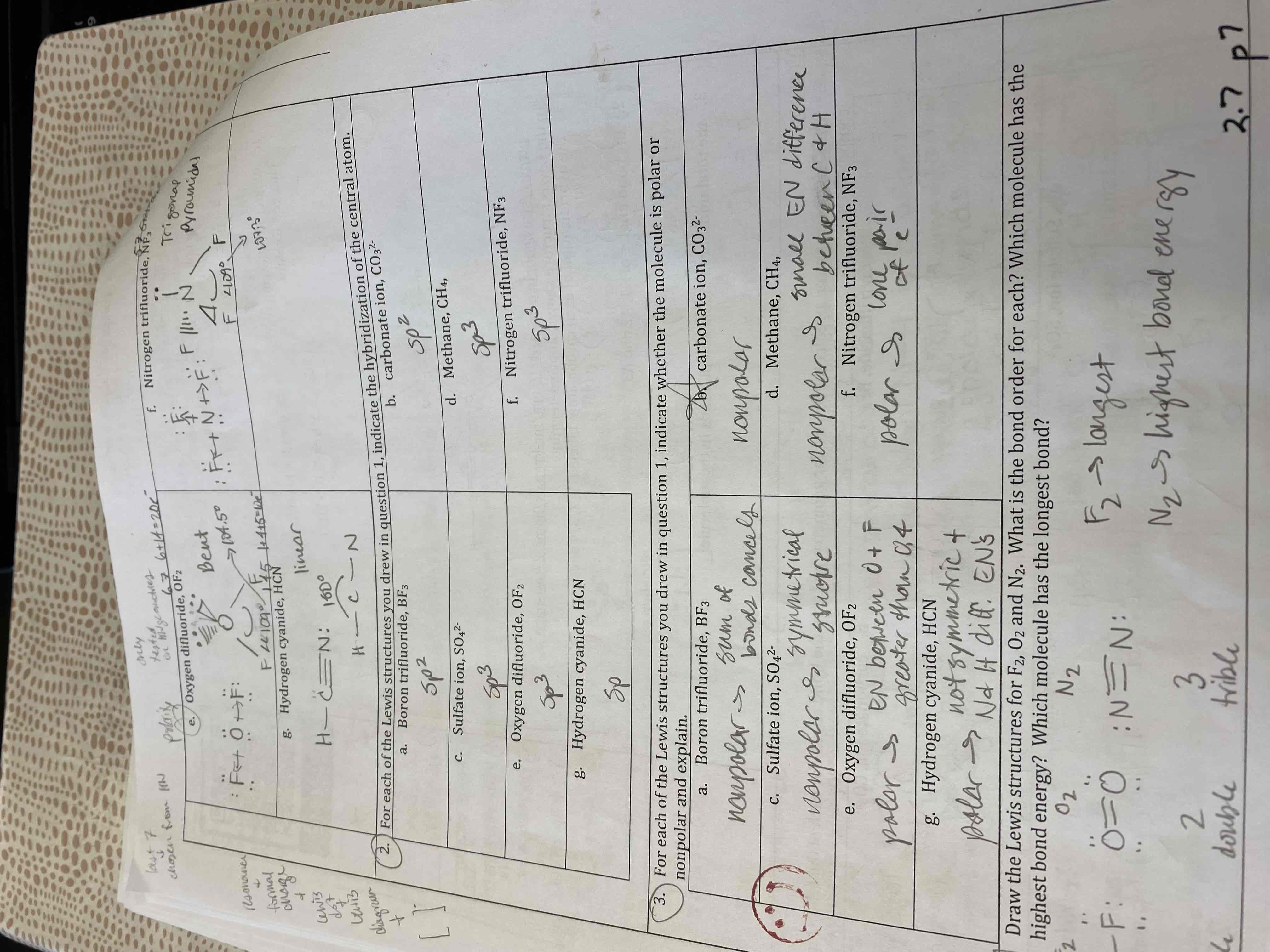
Draw Lewis structures, predict the molecular geometry, give the bond angles, specify the hybridization, and determine the polarity for each of the following compounds:
A) BF3
B) CO3²-
C) SO4²-
D) CH4
E) OF2
F) NF3
G) HCN