Complement, pattern recognition and phagocytes
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
What are the 2 main recognition pathways for complement?
Classical and Lectin
What is another complement pathway?
Alternative pathway
What are the effects of complement?
Immune cell recruitment
Opsonisation for phagocytosis
Cell lysis
Why is activation and regulation of these proteins important?
Deficiency can lead to increased susceptibility to infection (particularly bacterial infection)
Un-regulated/excess activation can lead to pathology
What are the 2 types of regulation?
Intrinsic and extrinsic
What is intrinsic regulation?
Determined by the intrinsic characteristics of complement cascade proteins themselves. The cascade can only be activated under specific circumstances
What is extrinsic regulation
Determined by other proteins – referred to as“regulators”. Their job is to stop the cascade, preventing inappropriate or excessive activation.
What is the classical pathway activated by?
IgM or IgG
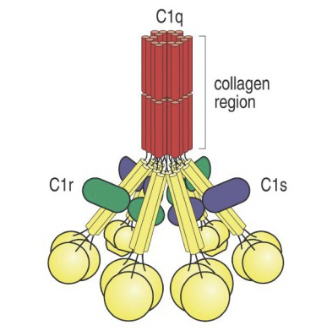
What is the structure of the C1 complex (classical pathway)?
What is the structure of mannose-binding lectin (lectin pathway)?
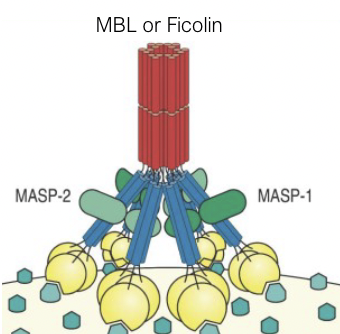
What is the lectin pathway activated by?
Mannose or NAG
How does the classical pathway work?
IgM binds target antigen
Conformational change from planar to spider-like
C1q binds IgM:Ag
Or
IgG binds target in regular patterns(PAMPs)- Spacing is important
C1q binds IgGn:Ag (n>1)
Example of intrinsic regulation
What happens once the C1q binding site os exposed?
C1q binds
Conformational change
Activation of C1R which cleaves itself and activates C1S
C1S can cleave and activate C4
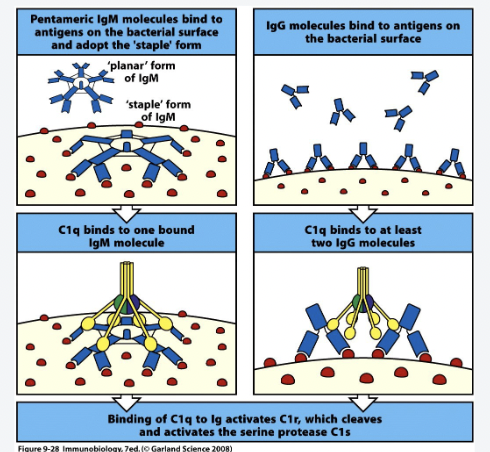
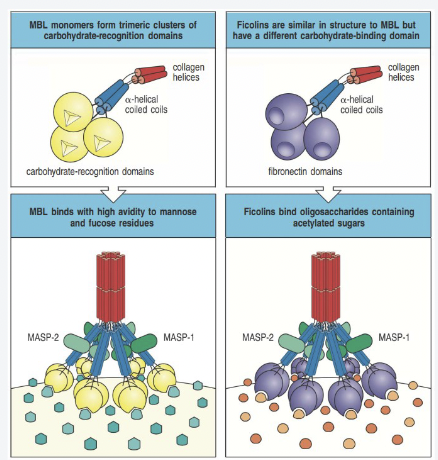
How does the lectin pathway work?
MBL or Ficolins bind target antigens in regular patterns (PAMPs)- Spacing is important
Conformational change of MBL
Allows activation of MASPs
MASPs can cleave/activate C4
What are proenzymes/zymogen?
Inactive enzyme
Cleavage, usually by another protease, results in change in conformation and exposure of the catalytic active site
This is a mechanism of intrinsic regulation
Proenzymes in the complement pathway are serine proteases
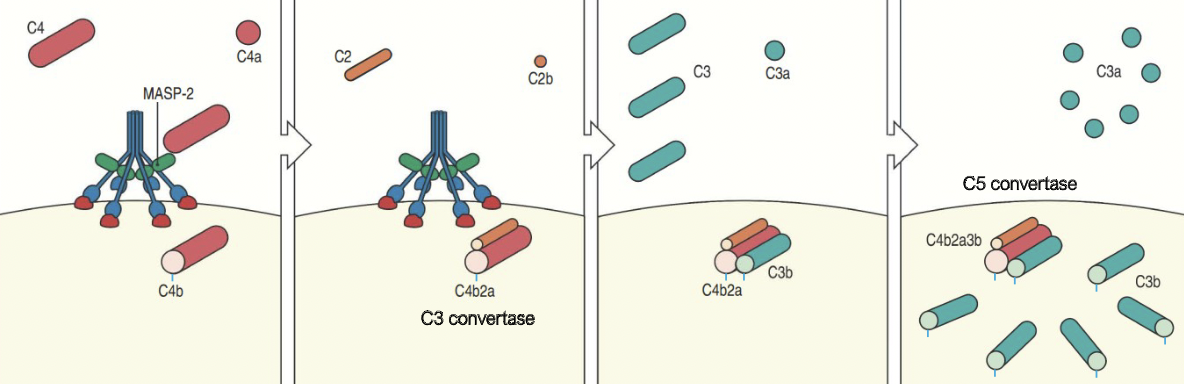
How does C3b opsonisation occur?
Activated by MASP-2 associated with MBL cleaves C4 to C4a and C4b which bind to microbial surface
C4b then binds to C2 which is cleaved by MASP-2 to C2a and C2b forming the C4b2a complex
C4b2a is an active C3 convertase cleaving C3 to C3a and C3b which binds to the microbial surface or to the convertase itself
One molecule of C4b2a can cleave up tp 1000 molecules of C3 to C3b.
Many C3b molecules bind to the microbial surface.
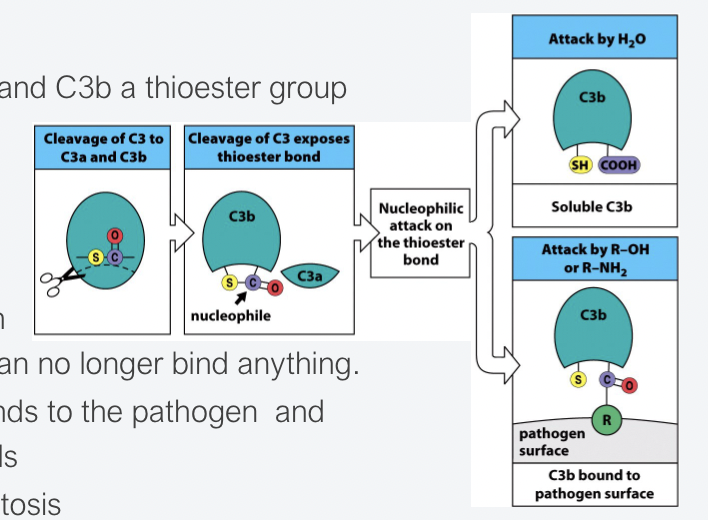
How do thioester domains become exposed and what happens?
When C3 is cleaved into C3a and C3b a thioester group is exposed.
The thioester group is super reactive therefore the half-life is short.
If it doesn’t bind to a pathogen instantly, it is hydrolysed and can no longer bind to anything.
This ensures that C3b only binds to the pathogen and not to neighbouring human cells
C3b opsonisation → Phagocytosis
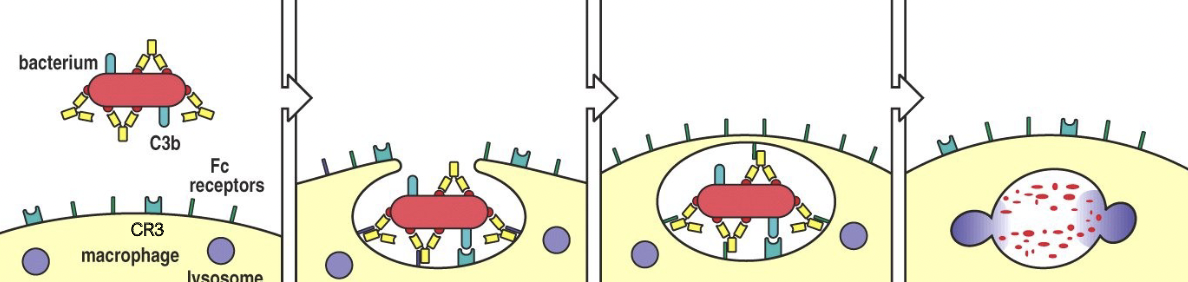
How does C3b cause opsonisation for phagocytosis?
C3b is deposited all over the target surface
C3b is an “eat me” signal which is recognised by Complement Receptor 3 (CR3) on phagocytic cells (Bacteria, apoptotic cells, immune complexes)
Which are the phagocytic complement receptors?
CR3 and CR4
How does complement-mediated phagocytosis work?
Bacterium os coated with complement and IgG antibody
When iC3b binds to CR3 and the antibody binds to Fc receptor, bacteria are phagocytosed.
Macrophage membranes fuse creating membrane-bounded vesicle, the phagosome
Lysosomes fuse with these vesicles, delivering enzymes that degrade the bacterium
What is the alternative pathway?
C3b deposited by classical or lectin pathway C3 convertase
C3b binds factor B
Bound factor B is cleaved by plasma protease factor D into Ba and Bb
C3bBb complex is a C3 convertase, cleaving many C3 molecules to C3a and C3b
C3 undergoes spontaneous hydrolysis to C3(H2O) which binds to factor B allowing it to be cleaved by factor D into Ba and Bb
The C3(H2O) complex is a C3 convertase, cleaving more C3 into C3a and C3b. C3b is rapidly inactivated unless it binds to cell surface.
Factor B binds non-covalently to C3b on a cell surface and is cleaved by Bb by factor D.
How are C5 convertase formed and what do they do?
C4bc2ac3b or C3bBbc3b complexes allow C5 convertase to cleave C5 into C5a and C5b
C5a is released = potent anaphylatoxin
C5b conformational change
Release from C5-convertase
Exposes binding site for recruitment of “terminal pathway components”
How does MAC pore formation occur?
Intrinsic regulation
Conformational changes upon binding next component
Exposure of lipophilic sites → membrane insertion
Only required for efficient killing of Neisseria bacteria (evident from people with Terminal pathway deficiencies)
All other bacteria C3b-opsonisation sufficient
What is MAC?
Membrane attack complex
How does immune cell recruitment occur?
C3a and C5a are anaphylatoxins
They attract and activate cells expressing C3a and C5a-Receptors (7TM GPCRs)
Chemotaxis: attract phagocytes to site of infection
Cell activation
Mast cells → release of pro-inflammatory content
Phagocytes → enhance phagocytosis and intracellular killing
Immune cells → enhance secretion of cytokines and prostaglandins
Excess activation results in inflammation
What is a C5a chemotactic gradient?
How does extrinsic regulation work?
What diseases are associated with complement?
Infectious diseases
Paroxysmal nocturnal hemoglobinuria
Psoriasis
Myasthenia gravis
Cancer
What does CD59 do?
Binds to C5b678 and prevents binding of C9
What is paroxysmal nocturnal haemoglobinuria?
Symptom of PNH = dark urine caused by haemoglobin.
Presence of haemoglobin in blood is caused by cell lysis by MAC.
Patients are deficient in both CD59 and DAF –these are GPI-anchored proteins.
Caused by a mutation in PIG-A – the enzyme responsible for attaching the GPI-anchor
What is innate immunity?
Pre-existing components which don’t change over time
Fast
Different molecules distinguish bacteria vs virus and can “steer” appropriate secondary immune response, but not tailored for e.g., COVID vs Influenza virus
Same response time and magnitude each time
What macrophages are in the liver?
Kupfer cells
What macrophages are in the brain?
Microglia
What macrophages are in the skin?
Langerhans cells
What are dendritic cells?
Takes up antigen in infected area (pinocytosis)
Transports to nearest lymph node
Presents it to T-cells to generate adaptive immunity
WHat are the different types of molecular pathogens?
Pathogen Associated Molecular Patterns – PAMPs
Damage Associated Molecular Patterns – DAMPs
What are the features of PAMPs?
May be sugars, proteins, lipids, nucleic acids
Generally repetitive patterns
Are widely expressed on pathogens but not on intact human cells
What are receptors that recognise patterns called?
Pattern recognition receptors
What are the features of viral PAMPs?
Viruses have their genetic material in different ‘formats’ than human cells and in unusual locations
Viral genome: dsDNA,ssDNA,dsRNA,ssRNA
Unmethylated DNA CpG dinucleotides repeats
PRRs and their compartmentalisation
The type and location of the PRR determines the downstream signalling pathways and can broadly shape the whole immune response
Bacteria are mostly extracellular and when they’re internalised by host cells they exist in endosomes
Virus location depends on route of entry but usually endosome or cytoplasm
What do toll like receptors do?
Toll like receptors (TLRs) are signalling receptors
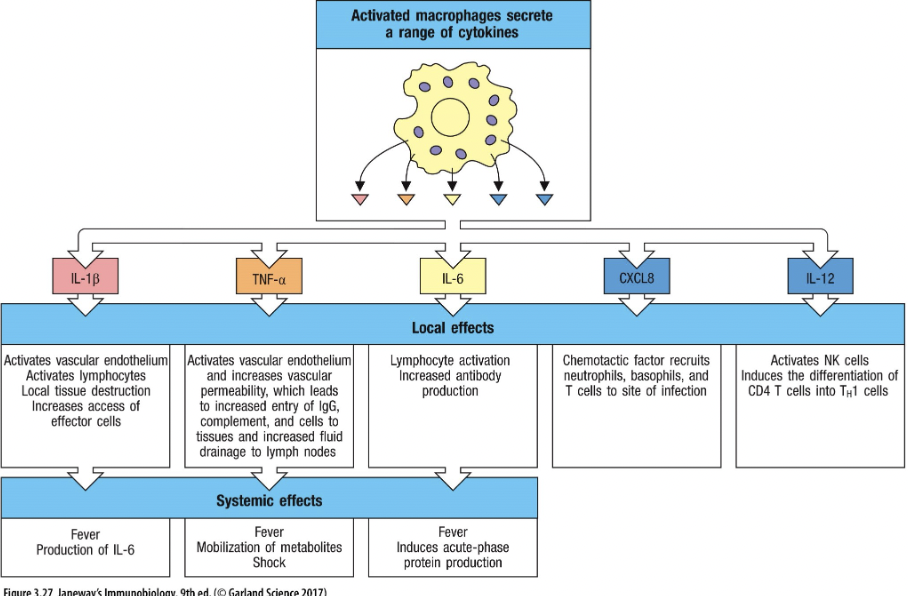
Extracellular TLRs detect bacterial product→ cytokine secretion, including TNFα, IL-1β, IL-6, IL-8 (more on these later)
Intracellular TLRs detect viral products → cytokine secretion inc. IFNs.
What is PRR function?
Soluble forms
Mannan Binding Lectin (MBL)
Ficolin
C1q
C-reactive protein (CRP)
Membrane bound
Toll-like receptors (TLRs) intracellular and extracellular
Lectins: carbohydrate recognition
Scavenger receptors
What are the features of cytokines?
Produced by immune cells AKA leukocytes – hence “interleukins” (IL)
Protein chemical messengers made by immune cells that influence the behaviour of other cells, particularly immune cells
Proliferate
Activate cells
Induce phagocytosis
Induce intracellular killing
Induce expression of receptors
INFLAMMATION
What is chemotaxis?
Cell migration
How do immune signals cause chemotaxis?
Complement cascade → production of anaphylatoxins (C3a and C5a) recognised by complement receptors
Chemokines recognised by chemokine receptors on immune cells
E.g. IL-8 secreted by macrophages and endothelial cells
How do pathogens cause chemotaxis?
Formylated Peptides bind the FPR or fMet-Leu-Phe (fMLF) receptor
Bacteria protein synthesis is initiated with a formylmethionine (fMet)which can be shed by bacteria