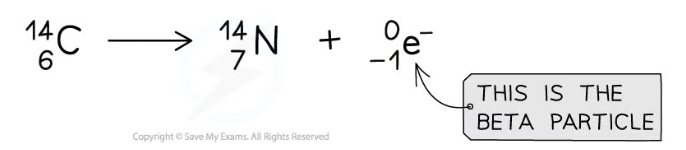4.2.5 - Beta Decay
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
What happens during beta decay ?
A neutron changes into a proton and an electron
What happens to the electron ?
It is emitted and the proton remains in the nuclei
What is formed ?
A completely new element is formed because the atomic number changes
What is a beta particle ?
It is a high-speed electron
What mass does the beta particle have ?
0
Why is this ?
This is because the electron has a negligible mass, compared to neutrons and protons
What happens to the mass number ?
The mass number of the decaying nuclei remains the same
What is the atomic number of electrons ?
They have an atomic number of -1
What does this mean ?
This means that the new nuclei will increase its atomic number by 1 in order to maintain the overall atomic number before and after decay
Beta decay equation