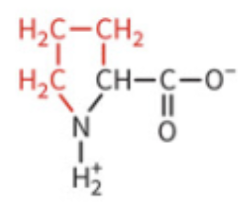
Amino Acids
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
alanine
nonpolar side chain
hydrophobic
no H-bond
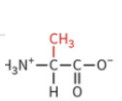
valine
nonpolar side chain
hydrophobic
no H-bond
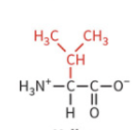
leucine
nonpolar side chain
hydrophobic
no H-bond
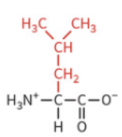
isoleucine
nonpolar side chain
hydrophobic
no H-bond
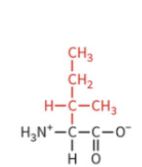
methionine
nonpolar side chain
hydrophobic
no H-bond
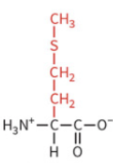
phenylalanine
nonpolar side chain
hydrophobic
no H-bond
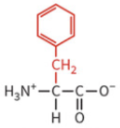
tryptophan
nonpolar side chain
hydrophobic
no H-bond
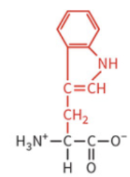
aspartate
polar: negatively charged
typically deprotonated at physiological pH
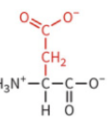
glutamate
polar: negatively charged
typically deprotonated at physiological pH
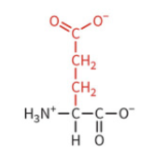
lysine
polar: positively charged
pKa 10.5
protonated and positively charged at physiological pH
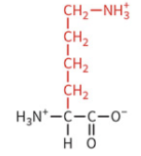
arginine
polar: positively charged
pKa 12.5
protonated and positively charged at physiological pH
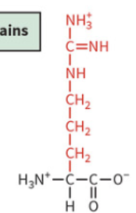
histidine
polar: positively charged
pKa 6.0
partially protonated at physiological pH
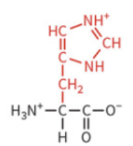
serine
polar: uncharged
hydrophilic
not typically ionized at physiological pH
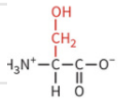
threonine
polar: uncharged
hydrophilic
not typically ionized at physiological pH
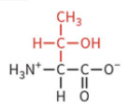
glutamine
polar: uncharged
hydrophilic
not typically ionized at physiological pH
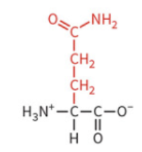
asparagine
polar: uncharged
hydrophilic
not typically ionized at physiological pH
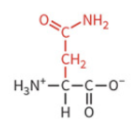
tyrosine
polar: uncharged
hydrophilic
not typically ionized at physiological pH
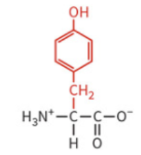
glycine
only H atom as side chain
not chiral
can reside in sites where two polypeptides come into close contact
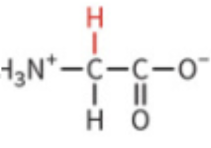
cysteine
polar, uncharged
can form covalent bond to make a disulfide link
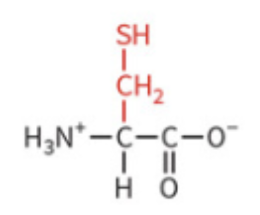
proline
hydrophobic character
can create kinks in polypeptide chains and disrupt secondary structure