Test 1: Alkanes through Conformations
0.0(0)
Card Sorting
1/91
Earn XP
Description and Tags
Does not include Functional Groups or Alkane Nomenclature
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
92 Terms
1
New cards
Hydrocarbons
Compounds containing only hydrogen and carbon (alkanes, alkenes, and alkynes)
2
New cards
Which of these reacts with hydrogen: Alkane, alkene, alkyne?
Alkene and Alkyne (hydrogenation, forms alkanes)
3
New cards
Constitutional or Structural Isomers
compounds with the same molecular formula that differ in the order in which the atoms are bonded to one another
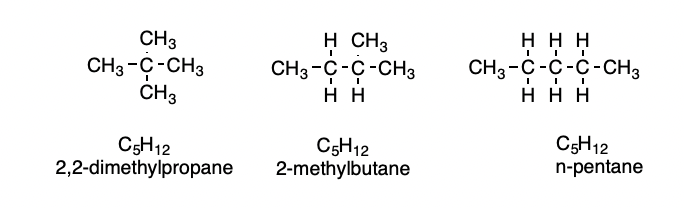
4
New cards
Saturated Hydrocarbons
Alkanes (only carbon-carbon single bonds)
5
New cards
Unsaturated Hydrocarbons
Alkenes, Alkynes, Arenes
6
New cards
Arenes
Hydrocarbons with one or more benzene-like rings
7
New cards
Boiling point, melting point, and density increase with ______ # carbons
increasing
(BP increases by 30°C with each additional CH2)
(BP increases by 30°C with each additional CH2)
8
New cards
Alkanes with 1-4 carbons are
gases
9
New cards
Alkanes with 5-17 carbons are
liquids
10
New cards
Alkanes with >17 carbons are
solids
11
New cards
Alkanes are not reactive (true/false)
true
12
New cards
Halogenation
Alkane + Halogen → alkyl halide + H(halogen) + other products (catalyzed by light)
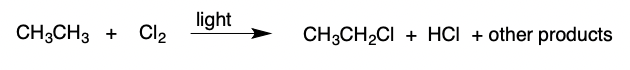
13
New cards
Oxidation of Alkanes
A reaction that either removes a hydrogen atom from a carbon or adds an electronegative element to the molecule (O, N, S, or a Halogen) → includes combustion reactions
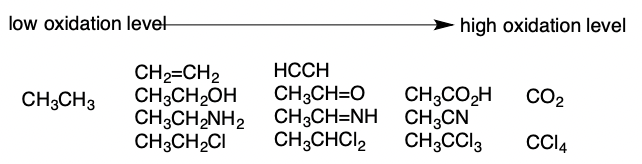
14
New cards
Combustion of Alkanes
Alkane + Oxygen → CO2 + H2O
(catalyzed by a spark)
\
Complete Combustion:
* yields CO2 and H2O
Incomplete Combustion:
* yields CO or just C and H2O
(catalyzed by a spark)
\
Complete Combustion:
* yields CO2 and H2O
Incomplete Combustion:
* yields CO or just C and H2O
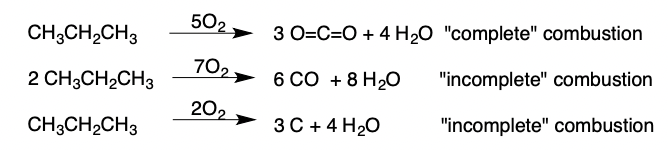
15
New cards
Heat of Combustion
Energy released when a compound is completely oxidized to CO2 and water
(depends mostly on the number of CH2 units: approximately 157 kcal/ methylene unit)
(depends mostly on the number of CH2 units: approximately 157 kcal/ methylene unit)
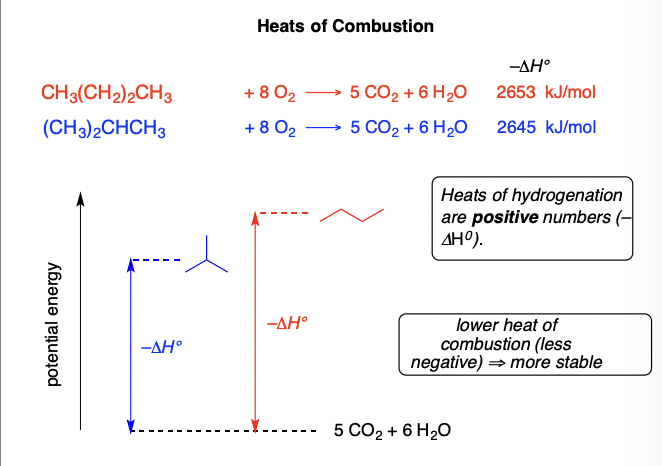
16
New cards
Explain ‘Like Dissolves Like’
A Solute will only dissolve in a solvent if both agree in terms of being Polar, Protic, or Donor molecules
\
Polar: has a dielectric constant > 15
Protic: can H-Bond (if there are H on O, N, F)
Donor: can donate an e pair from O or N
\
Polar: has a dielectric constant > 15
Protic: can H-Bond (if there are H on O, N, F)
Donor: can donate an e pair from O or N
17
New cards
Acidity Trends
1. (Charge Effect) positively charged atoms are more acidic
2. (Element Effect) acidity increases with elements going across and down the PT
3. (Hybridization Effect) acidity is greatest in molecules with high s-character (sp3< sp2 < sp)
4. (Resonance Effect) stronger acids have conjugate bases with charges delocalized by resonance
5. (Polar effect) Strong acids have a higher amount of electronegative atoms closer to the acidic oxygen (goes with charge effect)
18
New cards
Reduction of Alkenes and Alkynes
a reaction that either adds H atoms or removes an electronegative atom from the molecule
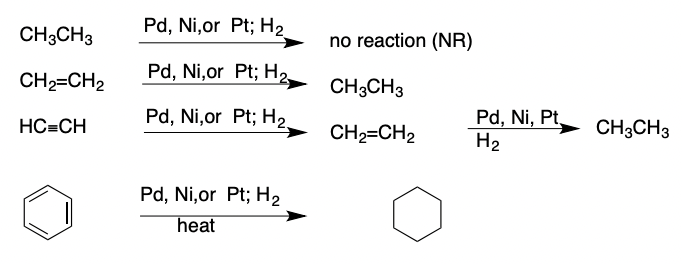
19
New cards
Steric Energy
(in an isolated molecule in gas phase at 0° K)
Relative energy of a conformation or stereoisomer calculated using classical mechanics
Relative energy of a conformation or stereoisomer calculated using classical mechanics
20
New cards
Stretch
(bond length)
Energy associated with stretching or compressing bonds from their optimal length
Energy associated with stretching or compressing bonds from their optimal length
21
New cards
Bend
(bond angle)
Energy associated with deforming bond angles from their optimal angle
Energy associated with deforming bond angles from their optimal angle
22
New cards
Stretch-Bend
Energy required to stretch two bonds involved in a severely compressed bond angle
23
New cards
Dipole-Dipole
Energy associated with the interaction of bond dipoles
24
New cards
Out of Plane
Energy required to distort a trigonal center out of planarity
25
New cards
Torsional Strain
Destabilization from eclipsing bonds on adjacent atoms
26
New cards
Van Der Waals Strain
Destabilization from two atoms being too close together
27
New cards
Dimensional Model
28
New cards
Newman Projection Model
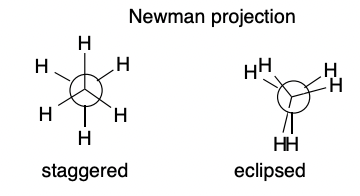
29
New cards
Graphical Model of ΔE when a molecule rotates about a single bond (ethane, in this example)
“E” is when the molecule is Eclipsed (highest energy)
“S” is when the molecule is Staggered (lowest energy)
“S” is when the molecule is Staggered (lowest energy)
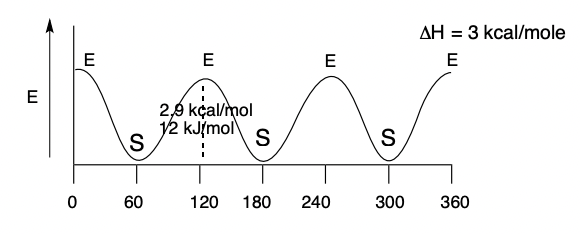
30
New cards
Eclipsed
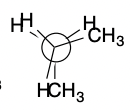
31
New cards
Gauche
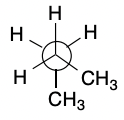
32
New cards
Anti
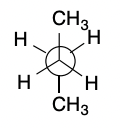
33
New cards
Substituted Ethanes
* an exception to the lowest energy conformation rule
* sometimes, a **gauche** conformation is preferred over staggered **if X and Y are electronegative substituents**
* sometimes, a **gauche** conformation is preferred over staggered **if X and Y are electronegative substituents**
34
New cards
Rotational Barriers
* increases with the number of CH3/H eclipsing interactions
* (with an electronegative middle atom, or a lone pair) the rotational barrier increases with the number of H/H eclipsing interactions (due to lone pair bubble colliding with them)
* (with an electronegative middle atom, or a lone pair) the rotational barrier increases with the number of H/H eclipsing interactions (due to lone pair bubble colliding with them)
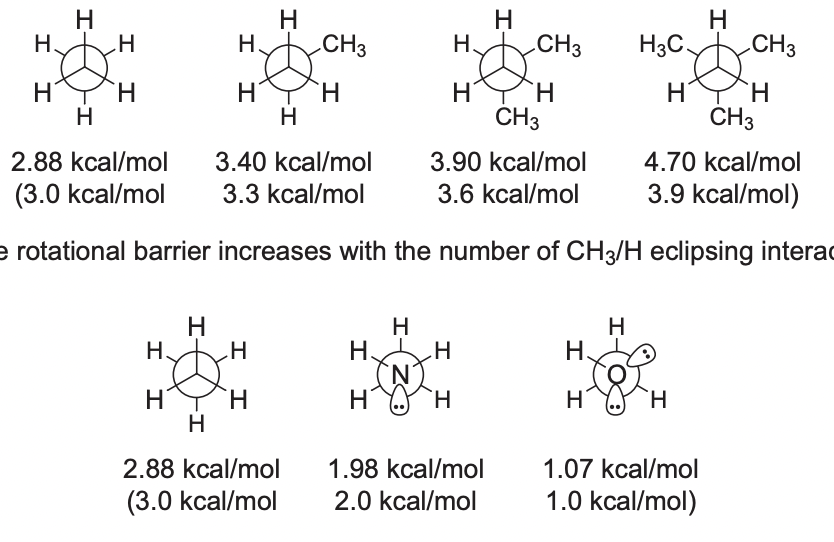
35
New cards
Relative Energies of Molecular Conformations
\
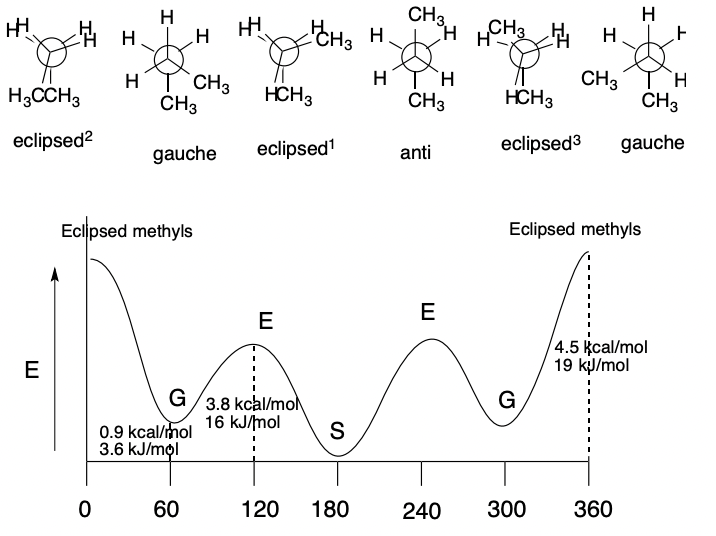
36
New cards
Cyclopropane
* Planar
* Steric Energy: \~128 kj/mol
* Bond angles: 60°
* Tetrahedral (sp3 bond angles): 109.5°
* Maximum overlap cannot be achieved
* Angle, Torsion, Van Der Waals
* Eclipsed (in photo)
* Steric Energy: \~128 kj/mol
* Bond angles: 60°
* Tetrahedral (sp3 bond angles): 109.5°
* Maximum overlap cannot be achieved
* Angle, Torsion, Van Der Waals
* Eclipsed (in photo)
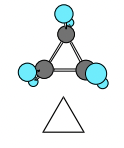
37
New cards
Cyclobutane
* Puckered
* Steric Energy: 122 kj/mol
* Angle (C-C-C: 88°), Torsion, Van Der Waals
* Partially Eclipsed (in photo)
* Steric Energy: 122 kj/mol
* Angle (C-C-C: 88°), Torsion, Van Der Waals
* Partially Eclipsed (in photo)
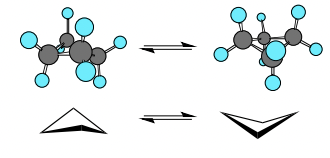
38
New cards
Cyclopentane
* Envelope
* steric energy: 48 kj/mols
* Torsion, Van Der Waals
* Partially Eclipsed (in photo)
* steric energy: 48 kj/mols
* Torsion, Van Der Waals
* Partially Eclipsed (in photo)
39
New cards
Puckering
allows bond angles to be at or close to the tetrahedral angle (109.5°) and minimizes torsional strain (electron-electron repulsions in eclipsed bonds) between adjacent C-H bonds
40
New cards
Chair Conformations
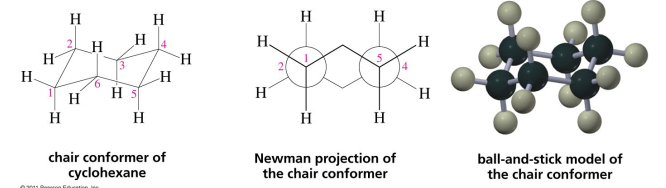
41
New cards
Equatorial Bonds
\
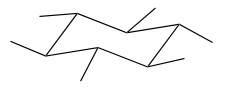
42
New cards
Axial Bonds
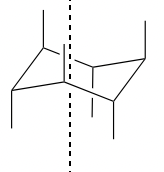
43
New cards
Half Chair
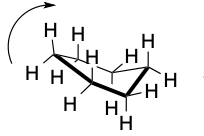
44
New cards
Boat
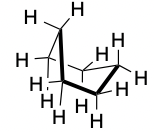
45
New cards
Relative Stability of Chair, Half-Chair, and Boat Conformations
High Energy
* half-chair
* boat
* twist boat
* chair
Low Energy
* half-chair
* boat
* twist boat
* chair
Low Energy
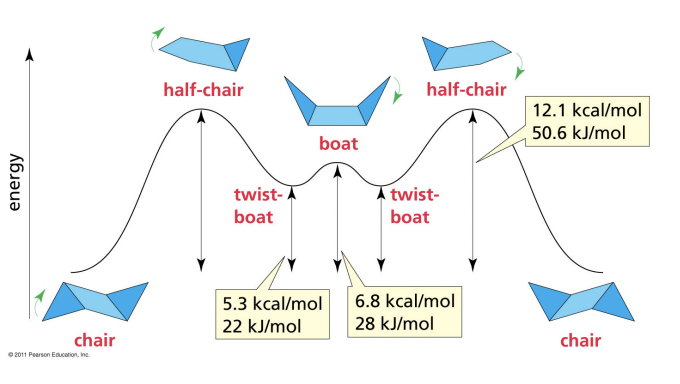
46
New cards
Half chair conformation
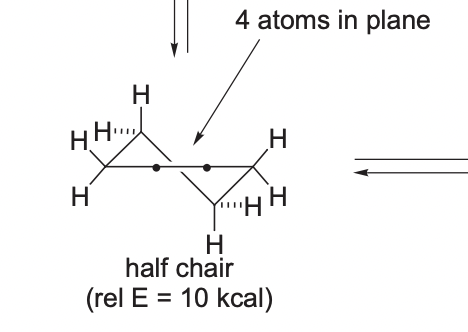
47
New cards
Twist Boat Conformation
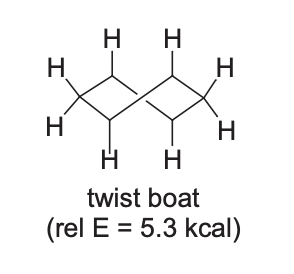
48
New cards
Substituents on cyclohexanes prefer to occupy _____ positions
Equatorial positions due to 1,3 diaxial interactions
49
New cards
Compound Newman Projection Models: Anti
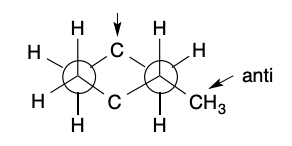
50
New cards
Compound Newman Projection Models: Gauche
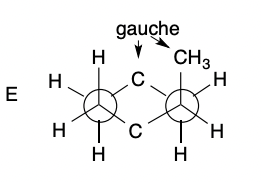
51
New cards
Relationship of Equatorial/axial percentage of possibility positions to K
K = \[equatorial %\]/\[axial %\]
\
(ex in photo) K = 95/5 = 19
\
(ex in photo) K = 95/5 = 19
![K = \[equatorial %\]/\[axial %\]
\
(ex in photo) K = 95/5 = 19](https://knowt-user-attachments.s3.amazonaws.com/1ce04b78ebdb449ca8dee191d86c831c.jpeg)
52
New cards
Gibbs Free Energy
ΔG = -RT(lnK)
53
New cards
Disubstituted Cyclohexanes
two substituents on a cyclohexane
\
Cis:
* substituents on the same side of the ring
Trans:
* substituents on opposite sides of the ring
\
Determine cis/trans stability by calculating ΔG°
\
Cis:
* substituents on the same side of the ring
Trans:
* substituents on opposite sides of the ring
\
Determine cis/trans stability by calculating ΔG°
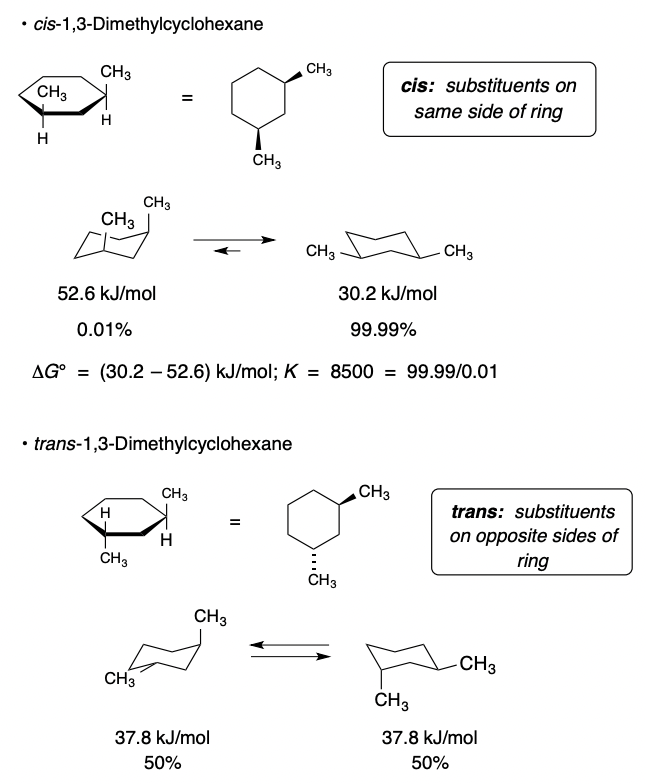
54
New cards
Bicyclic Compounds
* Conformationally locked (chairs cannot flip back and forth between conformations)
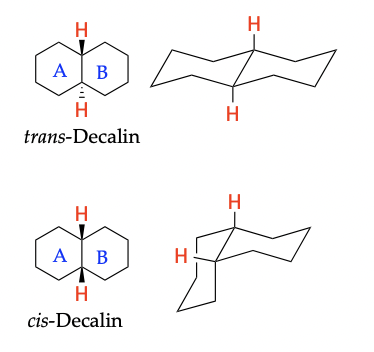
55
New cards
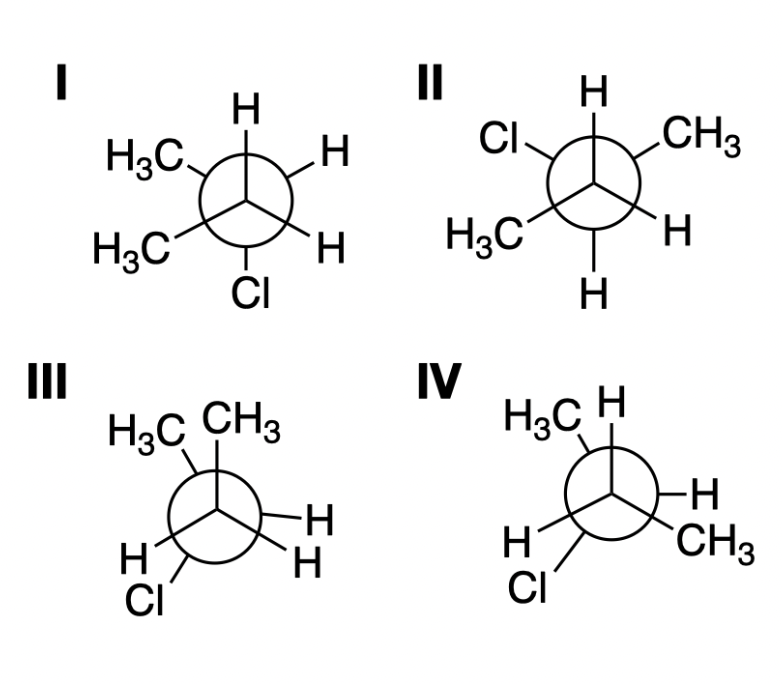
Rank the stability of these Newman Projections
III < IV < I < II
\
* Eclipsed conformers are less stable than staggered conformers due to torsional strain
* III < IV because there is more steric strain due to eclipsing methyl groups
* I < II because of the higher steric strain of the gauche methyl groups
\
* Eclipsed conformers are less stable than staggered conformers due to torsional strain
* III < IV because there is more steric strain due to eclipsing methyl groups
* I < II because of the higher steric strain of the gauche methyl groups
56
New cards
Drawing Alternate Cyclohexane structures
* chair inverts (over x axis)
* axial substituents become equatorial
* equatorial substituents become axial
* all substituents will maintain the direction they are facing (up stays up, down stays down)
* axial substituents become equatorial
* equatorial substituents become axial
* all substituents will maintain the direction they are facing (up stays up, down stays down)
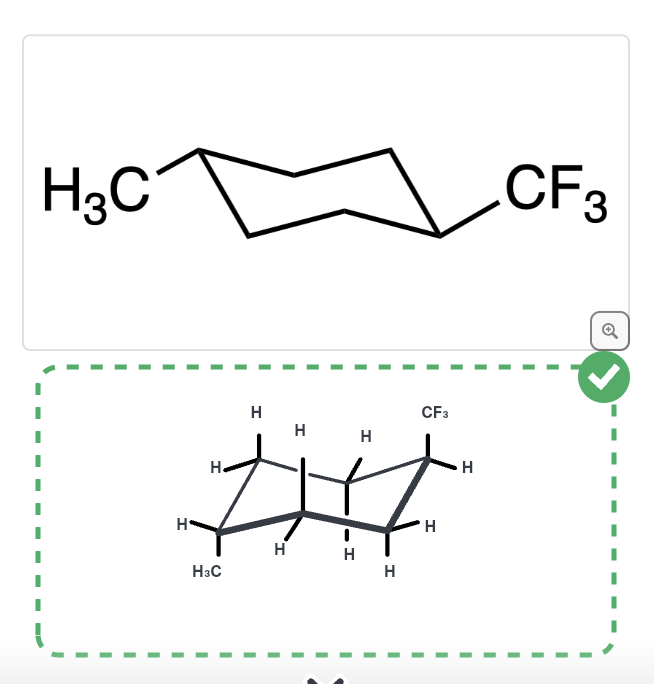
57
New cards
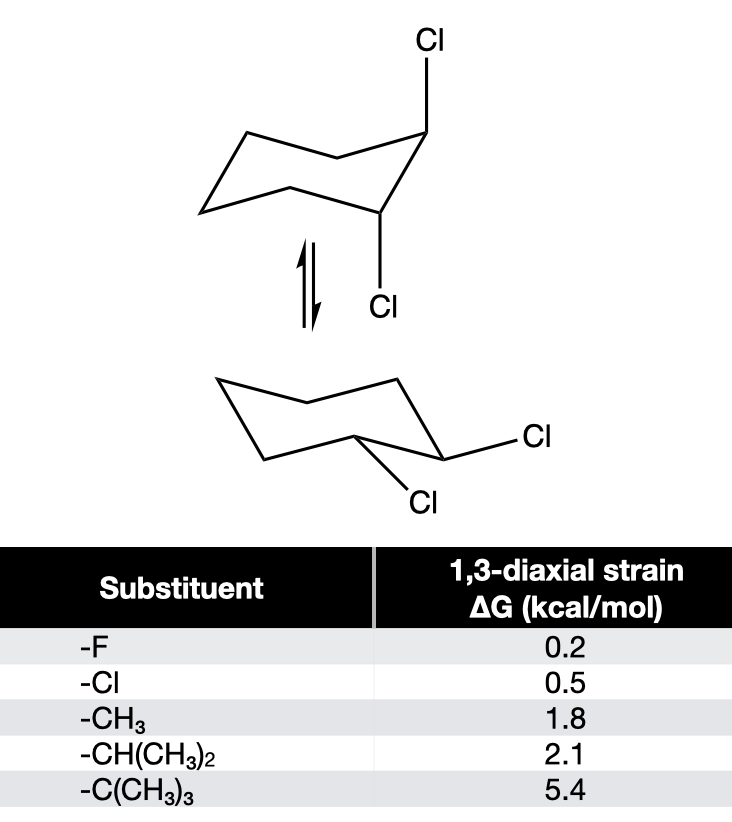
Calculate the difference in ∆G due to 1,3 diaxial strain between these two structures given A-values
1\.0 kcal/mol
58
New cards
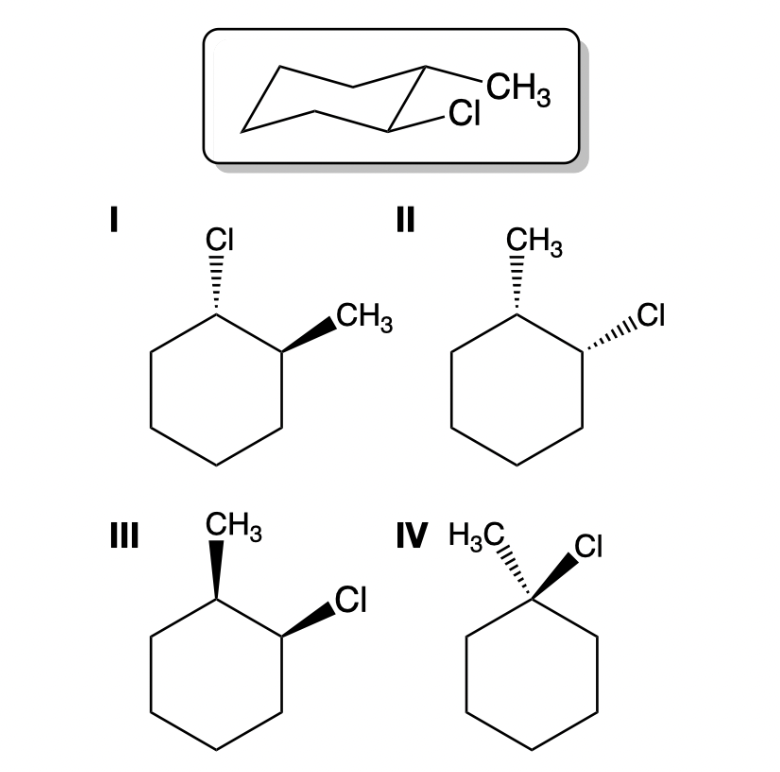
Which 2D figure represent the chair structure?
I
59
New cards
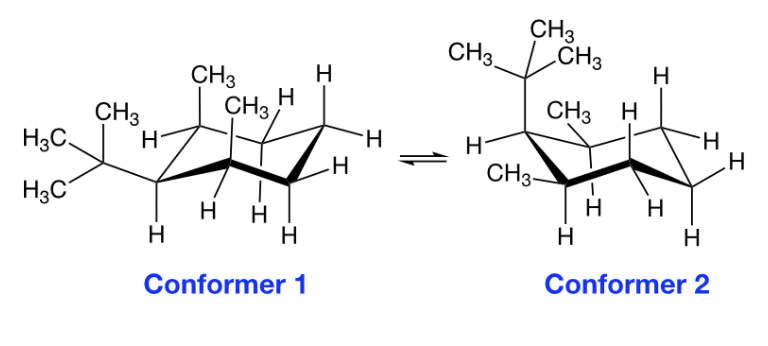
Which conformer is more stable?
Conformer 1
60
New cards
Bronsted-Lowry Acid
Proton Donor
61
New cards
Bronsted-Lowry Base
Proton Acceptor
62
New cards
Strong Acid
completely ionizes in water (have weak conj. bases)
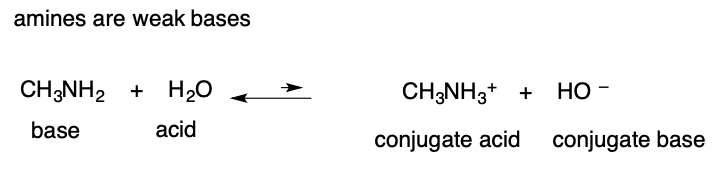
63
New cards
Weak Acid
only partially dissociated in water (have strong conj. bases)
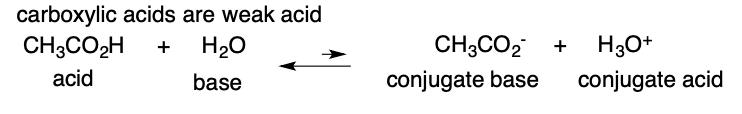
64
New cards
As acid strength increases, the basicity of the conj. base _______
decreases
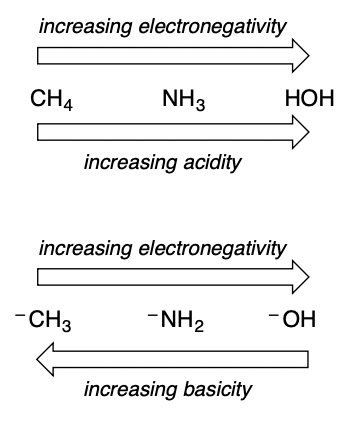
65
New cards
Low pKa means
high acidity, the better ability of an atom to stabilize a negative charge
66
New cards
Acidity Constant (Ka)
\[A-\]\[H+\]/\[AH\]
the greater Ka, the more acidic
the greater Ka, the more acidic
![\[A-\]\[H+\]/\[AH\]
the greater Ka, the more acidic](https://knowt-user-attachments.s3.amazonaws.com/e5887c18416f4e139292a099db52d7ee.jpeg)
67
New cards
pKa
\-log\[Ka\]
68
New cards
Equilibrium favors the side with the ________ acid
weaker
69
New cards
Lewis Acid
electron pair acceptor (any species with an electron-deficient atom)
70
New cards
Lewis Base
electron pair donor (any species with an unshared pair of electrons)
71
New cards
Nucleophile
electron-rich atom, often negatively charged, with a free lone pair to donate to another atom
72
New cards
Electrophile
electron-poor atom with a low-lying vacant or easily vacated orbital; wants to accept electrons from a nucleophile
73
New cards
Relative importance of resonance structures
Most Important
1. all octets are filled
2. negative charges exist on the most electronegative atoms
3. charge separation is minimized
1. all octets are filled
2. negative charges exist on the most electronegative atoms
3. charge separation is minimized
74
New cards
Atomic Orbitals
unhybridized orbitals on an atom (s, p, d)
75
New cards
Linear combination of atomic orbitals
individual wave functions (orbitals) combine to form hybrid atomic orbitals (sp, sp2, sp3) and molecular orbitals (*σ, σ*, π, π*)*
76
New cards
Hybrid Atomic Orbitals
Combination of atomic orbitals from the __**same**__ atom
77
New cards
Molecular orbital
combination of atomic orbitals from __**different**__ atoms
78
New cards
Conservation of Orbitals
when you add orbitals together, you always end up with the same amount of orbitals that you started with
79
New cards
bonding
(+/+ or -/-) electron density centered between nuclei
80
New cards
anti-bonding
(+/-) generally has a node between nuclei
81
New cards
node
an area of zero electron density
82
New cards
In stable bonding situations, usually only the ______ and _________ orbitals are occupied
sigma, pi
83
New cards
sigma bonding orbitals
* cylindrically symmetrical molecular orbitals
* electron density is centered along the axis of the bond
* single bonds = sigma bonds
* electron density is centered along the axis of the bond
* single bonds = sigma bonds
84
New cards
pi bonding orbitals
* not cylindrically symmetrical
* electron density is located above and below the axis of the bond
* double and triple bonds = pi bonds
* electron density is located above and below the axis of the bond
* double and triple bonds = pi bonds
85
New cards
Structure
determining the way in which atoms are put together in space to form complex molecules
86
New cards
Mechanism
understanding the reactivity of molecules: how and why chemical reactions take place
87
New cards
Synthesis
building complex molecules from simple using chemical reactions
88
New cards
Bond lengths
dependent on atomic size, bond order, and hybridization
89
New cards
Multiple Bonding
bond length is determined by bond order (Single > Double > Triple)
90
New cards
Effect of Hybridization on Length of Single Bonds
C-H and C-C bonds shorten slightly with increased S character on Carbon (sp > sp2 > sp3)
91
New cards
Bond dissociation energies
* energy for homolytic bond cleavage to uncharged radical fragments
* dependent on the specific molecular structure
* dependent on the specific molecular structure
92
New cards
Bond Strengths
* bond energies for a certain bond averaged over many different molecules
* for multiple bonds: strength is for single < double < triple bonds
* for multiple bonds: strength is for single < double < triple bonds