Unit 2 AP Chem Vocab
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
Covalent Bonds
2 NONMETALS
share electrons.
Non polar Covalent Bonds
Sharing electrons equally
Has a electron negativity difference of (<0.5)
Polar Covalent Bond
Share electrons unequally
One atom is hogging the electrons
Electron negativity difference is (0.5<)
The atom with the greater electron negativity is hogging the electrons
High potential energy
When the atoms are far apart
The period before they bond together
Low potential energy
When the atoms are close together
Bonding
Ionic Bonds
METAL + NONMETAL bond
The metal gives the electron away to the non metal
HIGHER melting point compared to covalent bonds
Metallic Bonding
Delocalized electrons (electrons are able to move freely within the structure
Good at conducting electricity

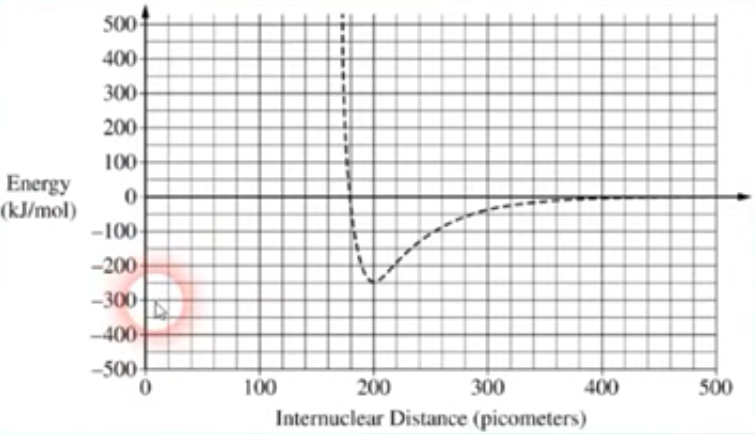
Covalent Bonding Graph —> Cl2
The bond length is where the distance between the 2 atoms is at its lowest
Bond length (I D) → 200
Bond Enthalpy (E) → 250 Kj/mole (ALWAYS POSITIVE)
Ionic Bonds/compounds —> Na + Cl
Na = 1s2 2s2 2p6 3s1
Cl = 1s2 2s2 2p6 3s2 3p5
Na gives one electron to Cl. Cl becomes a gains a octet → Na+ and Cl- →NaCl
This works because when the metal donates to the non metal, the opposite charges stick together because of their strong electrostatic attractions
Melting point in Ionic Bonds
Melting point increases as the magnitude of the charge increases
Because of Coulomb's law → LARGER charge = LARGER bond energy = Greater melting point
EX → Mg+2 and S-2 is stronger than Na+ Cl-
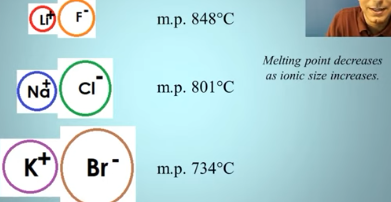
What if they are the same charge? (Melting points of ionic compounds)
melting point DECREASES as ionic size INCREASES.
When the ionic nuclei are far apart (due to size), the force of attraction gets weaker.
When predicting melting points you should…….
Look at the greater charge
Compare sizes (smaller Bonds = higher melting point)
Lattice energy
energy released when ions are combined into an ionic compound
STRONGER ionic forces (Based on size and charge) = Higher amounts of lattice energy released.
Lattice energy examples
LiF (small) → -1030 Kj/mol
NaCl (Medium)→ -746 kj/mol → Lower because there is not as much attraction compared to LiF
KBr (Large)→ -688 kJ/mol
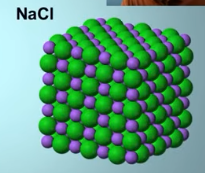
Ionic compounds
High melting point
Brittle (not malleable) (think salt NaCl)
Conducts electricity when dissolved in water (electrolytes)
Soluble in water (polar substances)
Has a repeating crystal structure (crystal lattice)
Compact — Not floating around
Crystal lattice
Repeating crystal pattern
Held together by electrostatic forces
Substitutional Alloys
Brass, Bronze, etc
Substitutes into the spot of other elements.

Interstitial Alloys
EX → Steel
Atoms that stick in between the small spaces of the main metal (hardening agent)
Allows them to stay in place making the compound stronger

Octet Rule
Most atoms are stable with 8 valence electrons
Exceptions to the Octet Rule
H is stable with 2 electrons
B is more stable with 6 electrons
Non metals with 3 or more energy levels can sometimes have more than 8 valence electrons (up to 10 or 12) → Expanded Octet.
When a central atom is bonded to more than 4 atoms, the central atom will have an expanded octet
Resonance structures
2 lewis electron dot structures which both represent correct structures for a molecule
Formal charge
A Way of assigning a charge to each individual atom in a molecule. To have it neutral, each should have a formal charge of 0.
Each dot is one
A bond gives 1 for each atom it is bonded to
Sigma Bond
Result of overlapping s orbitals
Pi Bonds
Result of overlapping p orbitals
Single bonds
Longest / Weakest → Sigma bond
Double Bonds
Middle → 1 sigma 1 pi
Triple Bonds
Shortest/ Strongest → 1 sigma 2 pi
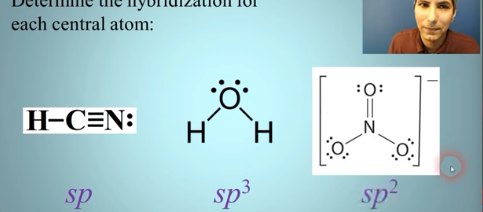
Hybridization
Largest sum → sp3d2
Add the number of sigma bonds to the number of unshared electron pairs on the atom
Between 2-6
Tetrahedral
4 sigma bonds
109.5 degrees
Trigonal Pyramidal
3 sigma bonds + 1 lone pair
107 degrees
The bond angle is smaller because the electron pairs exerts more repulsion than shared pairs
Bent
2 sigma bonds + 2 lone pairs
105 degrees
Trigonal planer
3 sigma bonds
120 degrees
Angular
2 sigma bonds + 1 lone pair
117 degrees
Linear
2 sigma bonds
180 degrees
Octahedral
6 sigma bonds
90 degrees
Square pyramidal
5 sigma bonds + 1 lone pair
90 degrees
Square Planar
4 sigma bonds + 2 lone pairs
90 degrees
T shaped
3 sigma bonds + 3 lone pairs
90 degrees
Trigonal Bipyramidal
5 sigma bonds
90 + 120 degrees
See-saw
4 sigma bonds + 1 lone pair
87 + 117 degrees
Trigonal Planer
3 sigma bonds + 2 lone pairs
120 degrees