Modulee 4 Part I
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Plasma protein
plasma: the complete straw-coloured fluid without cells
serum: straw-coloured fluid left without clotting factor
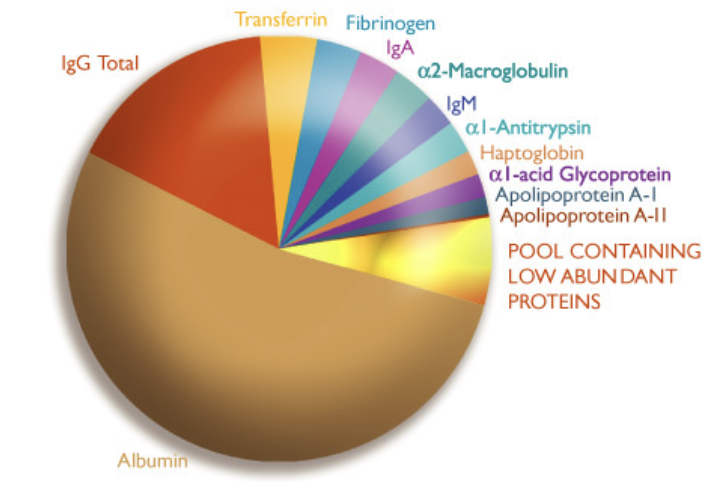
Plasma protein and their function
acid-base regulation
storage and transport
immune function
acute phase response
clotting
building and repairing tissue
enzymes
hormones
balance of water and electrolyte
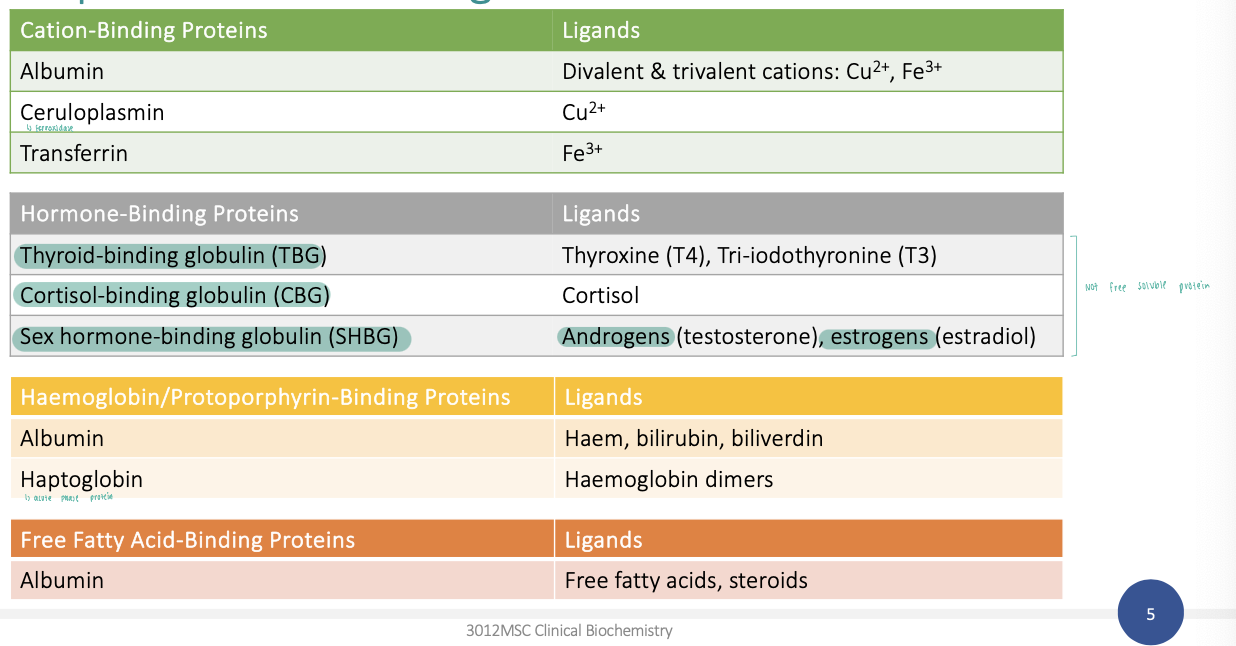
Transport proteins and ligands
Immunoglobulins
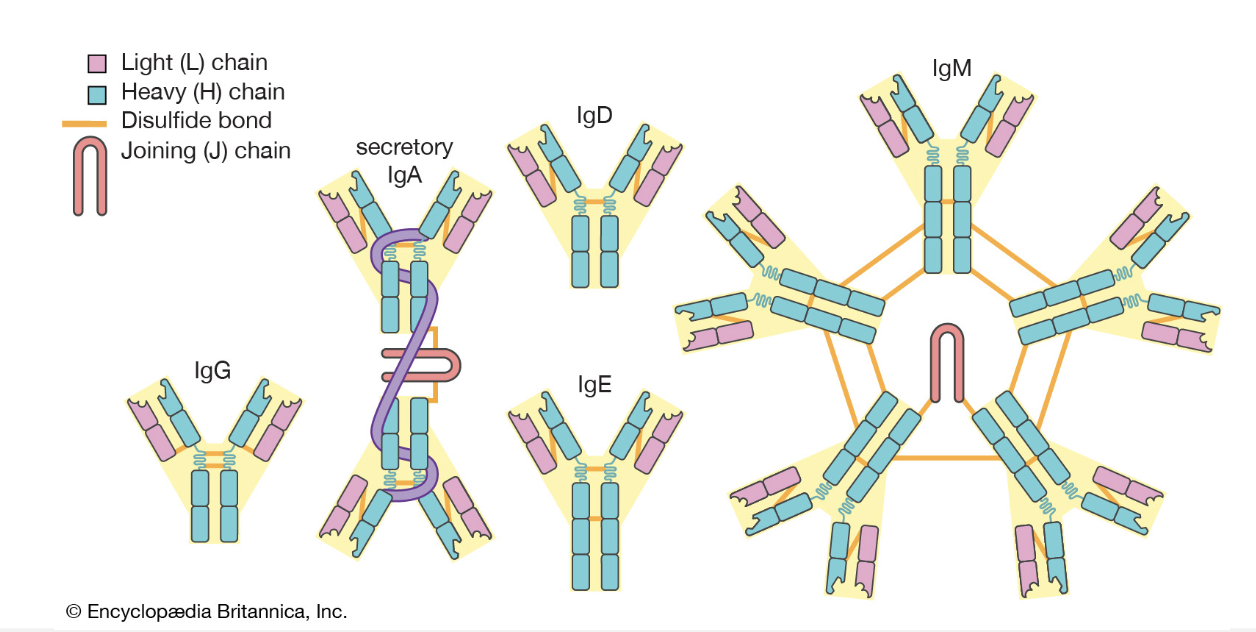
What are the functions of the 5 main immunoglobulins?
IgG: antiviral, antibacterial, protection of body spaces,
IgA: protection of tissue surfaces, gut, and respiratory tract.
IgM: antibacterial, antiviral
IgD: antigen receptor on B lymphocytes
IgE: allergic hypersensitivity
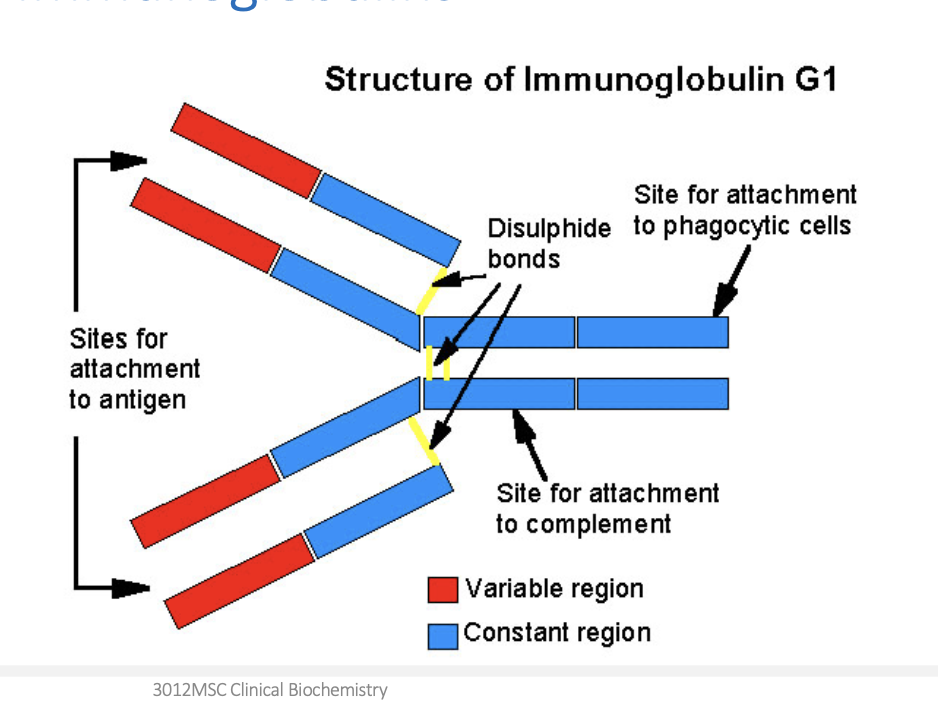
Protein structure
primary structure: the sequence of amino acids within the peptide or protein. the primary structure is held together by covalent bonds.
secondary structure: highly regular local sub-structures or domains, the two main types being the alpha-helix and the beta-pleated sheet
tertiary: three-dimensional folding of a single protein molecule; the alpha-helices and beta-pleated sheets are folded into a compact structure.
quaternary structure: a larger assembly of several protein molecules or polypeptide chains to form multimers.
Why are these proteins measured?
proteins that have physiological function in the circulation: a change in the level in plasma can be indicative of disease affecting circulation or the tissue that synthesizes the protein
proteins that leak from cell and tissues: a change in the level in plasma can be indicative of disease affecting tissue that synthesizes the protein
conditions that alter protein levels and enzyme activity in
changes in cellular proliferation
changes in cellular turnover or damage
changes in protein synthesis
inherited protein variants with altered activity
altered protein conformation
Enzymes as disease biomarkers
enzymes are catalytic proteins responsible for biological processes in all cells
cellular changes triggers the release of specific enzymes from affected tissues.
elevated enzyme levels can be measured in patient specimens such as serum, saliva, and urine.
enzymes characterized by substrate specificity and measurable activity can be used as diagnostic and prognostic biomarkers.
measurable changes in enzyme activity can serve as disease markers, often before clinical symptoms appear.
enzyme activity in plasma
Activity of enzyme in plasma
catalytic activity
balance of:
rate of synthesis
release form cell is proportional to rate of cell degradation.
rate of clearance.
What is proteomics?
it is concerned with the systematic, high-throughout apprach to protein expression analysis of a cell or an organism.
typical results of proteomics studies are inventories of the protein content of differentially expressed proteins across multiple conditions.
cells responds to internal and external changes by regulating the activity and level of its proteins; therefore… changes in the proteome provide a snapshot of the cell in action
proteomics enables the understanding the structure, function and interactions of the
what proteomics tells us about tissue specificity
proteins can have limited or widespread expression.
different isoforms of specific proteins can be present.
this information can be used to identify the cellular source of proteins in circulation.
Factors governing protein entry and removal from circulation
location of cells expressing protein: access to circulation?
subcellular localization of protein: cytoplasmic, membrane, or organelle?
in circulation: as a single protein or associated with other proteins/molecules?
half life: is the protein degraded?
excretion: is the protein filtered by the kidney? is it deposited elsewhere?
Measurement of total protein
quantitative formation of a violet-coloured complex between copper ions and peptide bonds in an alkaline medium; spectrophotometric endpoint or kinetic measurement at 540 nm.
usually adapted to automated analysis; has good specificity, accuracy, and precision; and has been proposed as the basis for the reference method.

Measurement of serum albumin
Bromocresol green
albumin beinds to BCG
absorbance at 628nm increases in proportion to protein concentration.
some non-specific absorbance changes with time
Bromocresol purple
albumin binds to BCP
absorbance at 603 nm increases in proportion to protein concentration.
more specific for albumin
albumin from animal sources do not bind to BCP in an equivalent manner to human albumin
Measurement of serum albumin —> Bradform Assay
Coomassie Brilliant Blue G-250
the red form of coomassie brilliant blue G-250 donates its free electrons to the ionizable groups (NH₃⁺) on protein
this causes disruption of proteins exposing its hydrophobic pockets
negatively charged coomassie brilliant blue G-250 binds to the protein and forms a stabilized blue form of the coomassie dye in proportion to the amount of protein.
the absorbance of the blue complex is measured at 595 nm
Measurement of Serum Albumin —> Lowey assay
Divalent copper ions in Folin-Ciocalteu reagent form a complex with peptide bonds at alkaline pH and are reduced to monovalent copper ions
Monovalent copper ion and the radical groups of tyrosine, tryptophan, and cysteine react with Folin reagent to produce an unstable product that becomes reduced to molybdenum/tungsten blue
Blue colour is proportional to the amount of tyrosine, tryptophan, and cysteine in the protein
Negatively charged Coomassie dye binds to protein and forms a stabilised blue form of the Coomassie dye. Absorbance of the blue complex is measured 650 nm
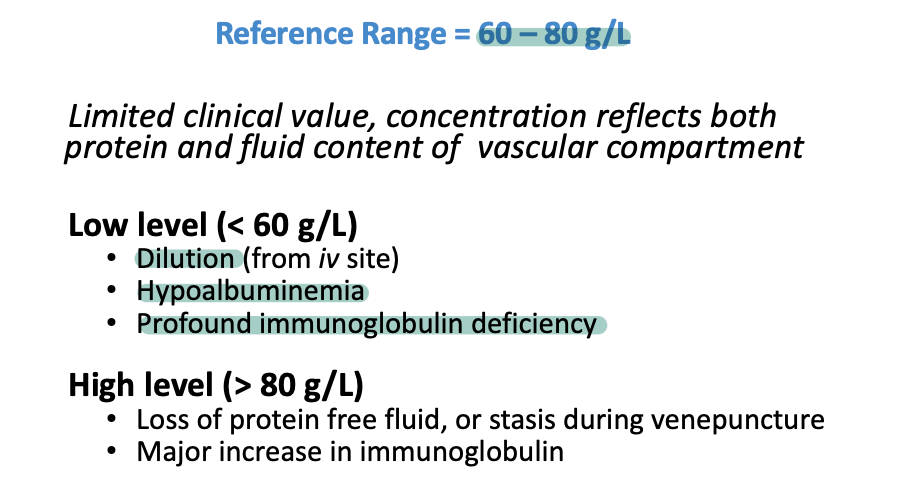
Measurement of total protein
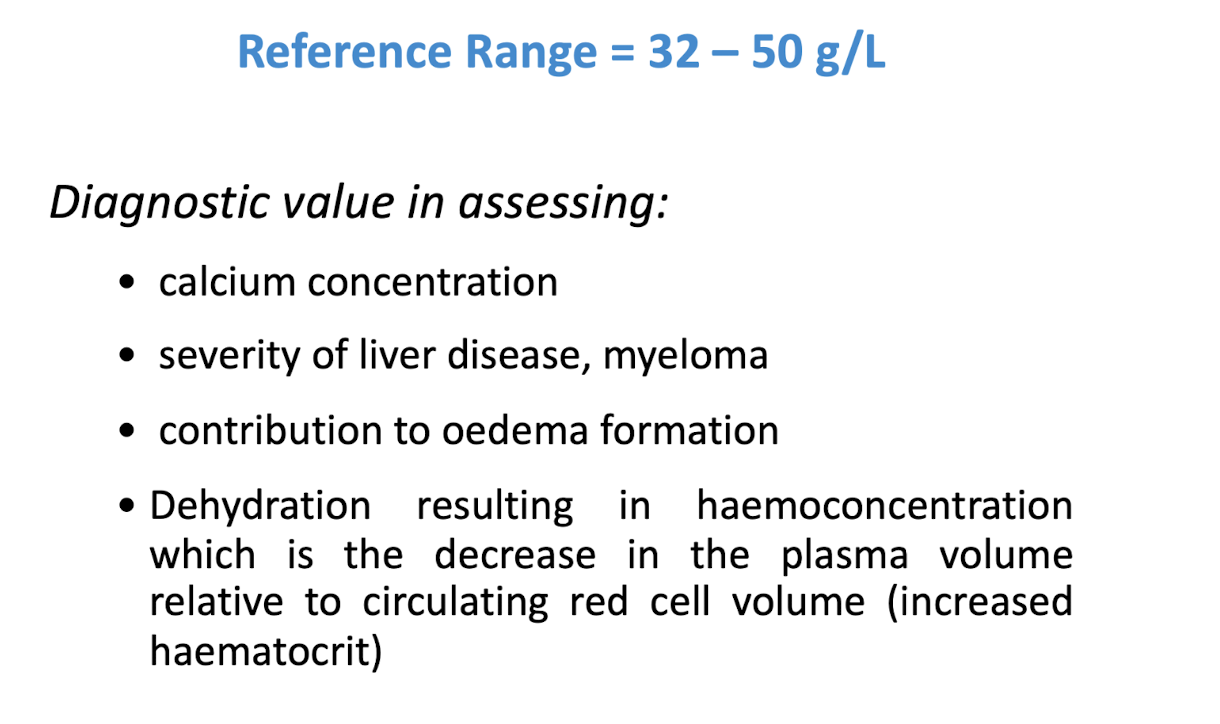
measurement of albumin
principles of protein electrophoresis ‘
migration of charged particles in a support medium due to an electric field
proteins are zwitterionic: they can be negative or positively charged depending on the pH of the solution.
migration depends on:
electric charge of the molecules
size and shape of the molecule
electric field strength
properties of the support material
temperature.
Uses of protein
Determining Molecular Weight: Estimating the size of proteins by comparing them to a molecular weight marker
Analyzing Protein Purity: Assessing the purity of protein samples by visualizing contaminating proteins
Protein Identification: Identifying proteins by comparing their migration patterns to known standards
Western Blotting: Transferring separated proteins to a membrane for specific detection using antibodies
Types of protein electrophoresis
Native PAGE: In native polyacrylamide gel electrophoresis, proteins are separated based on their native conformation and charge. Native PAGE is used for analysis of serum proteins
SDS-PAGE:In sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis, proteins are reduced to break disulphide bonds and denatured with SDS detergent. SDS unfolds proteins into linear chains and masks their
charged groups so they are separated based on their molecular weight.
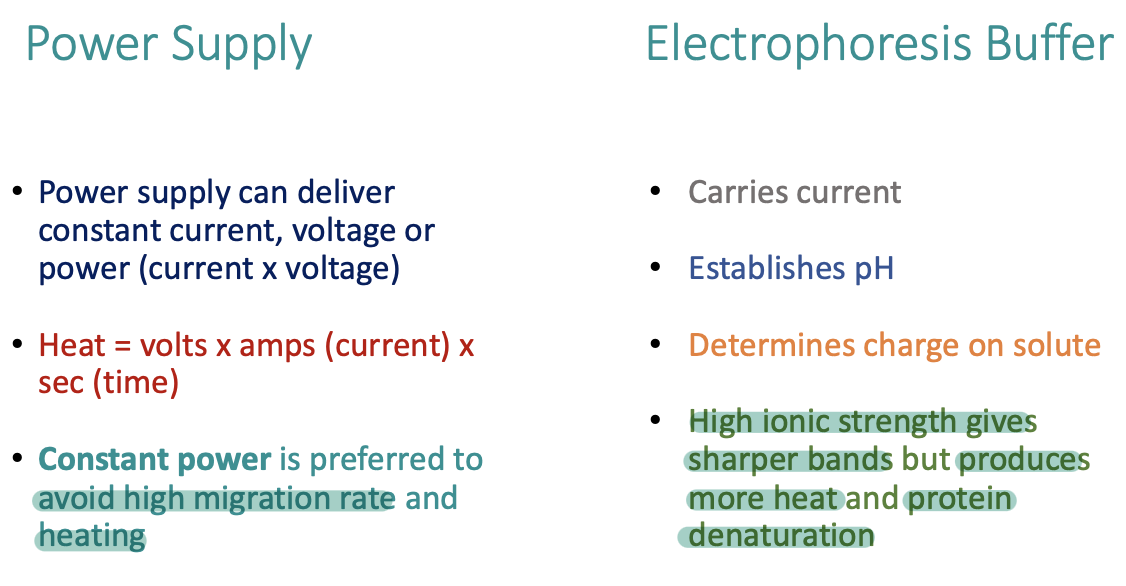
Power supply and electrophoresis buffer
Protein molecular weight measurement
Use of molecular weight standards to calibrate migration
Protein staining of gel after electrophoresis
Densitometry to measure the amount and migration of proteins
Plotting molecular vs Rf (migration distance divided by the migration distance of the dye front)
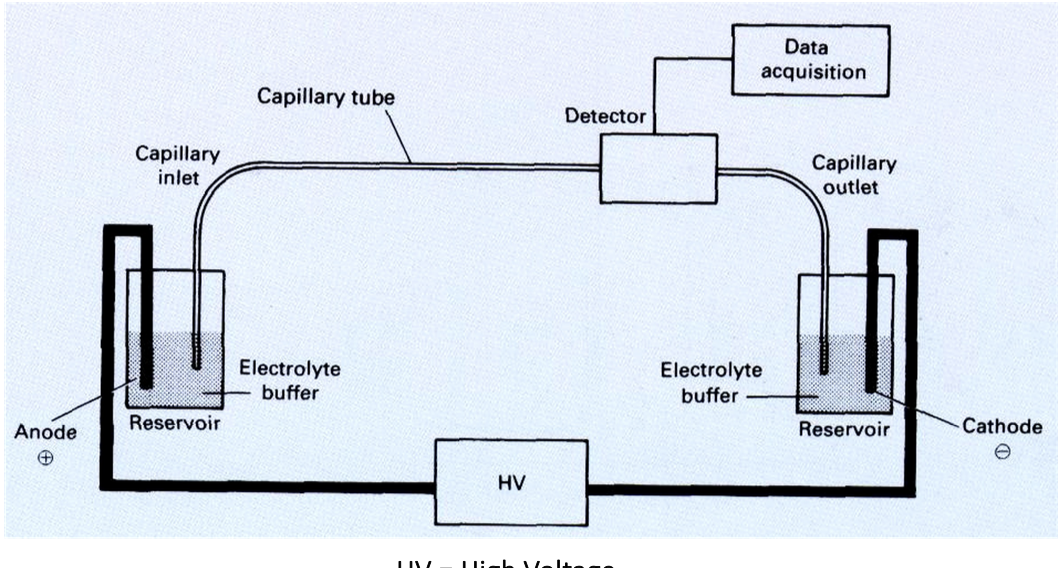
Capillary Electrophoresis
• Electrophoresis without support in narrow-bore tubing with
internal diameter of ~50 µm
• Movement of sample in buffer in high voltage electric field
• Separation of small or large molecules
- amino acids, nucleotides, peptides, proteins, nucleic acids
• Separation of DNA synthesis products:
- Oligonucleotide synthesis quality control
- Automated DNA sequence determination