20412- core organic chemistry
1/371
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
372 Terms
what is the preferred orientation in butane and why
staggered because there is a stabilising interaction between the filled C-h sigma bond and empty C-H sigma antibonding orbital whereas in the eclipsed conformation the filled orbitals repel

Describe the observation with ring opening and closing reactions
Trans conformers undergoes ring closing to an epoxide treatment whereas the cis diastereoisomer does not - the minor conformer is the reactive conformer
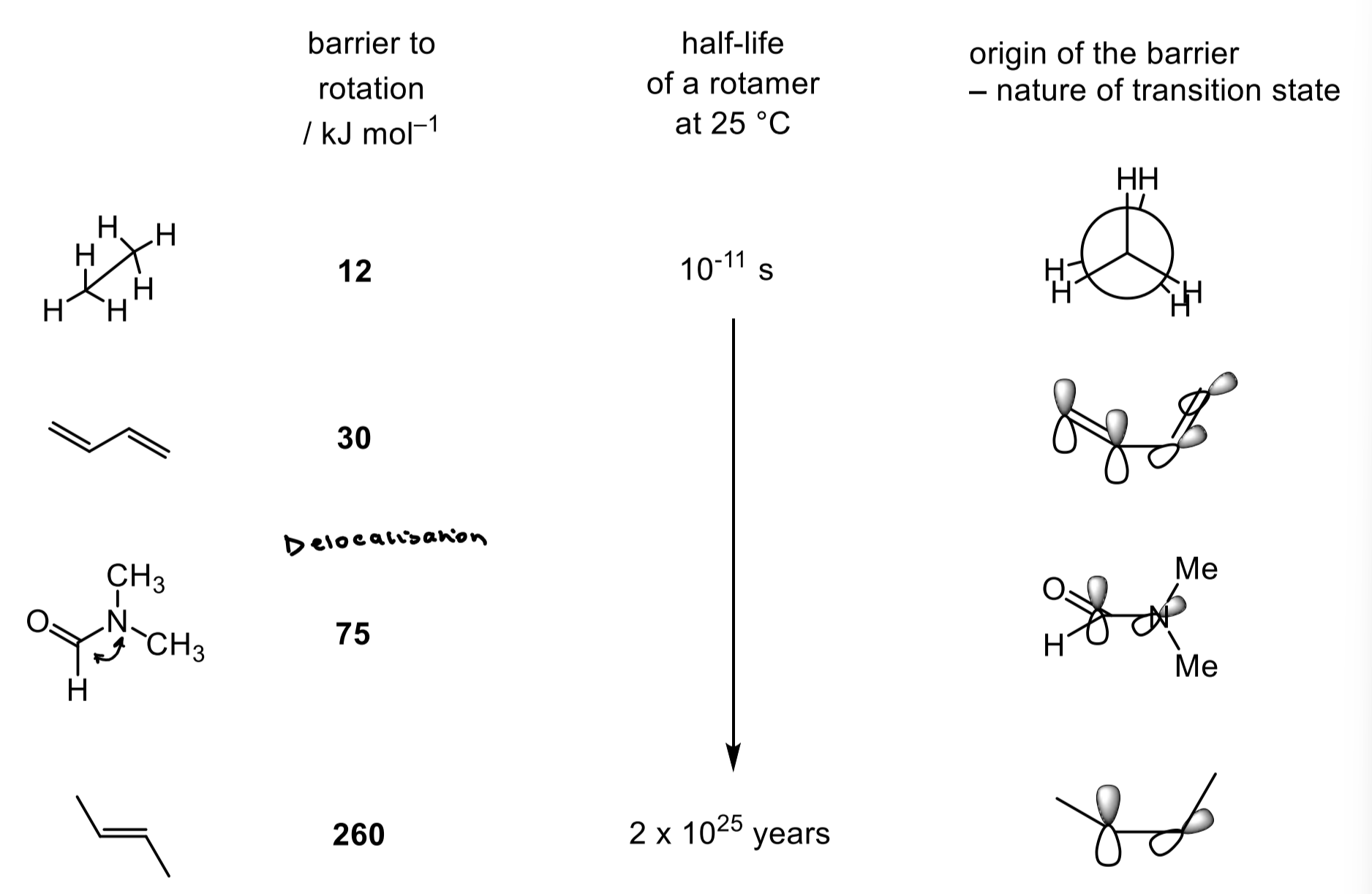
what things can affect rates and barriers
steric hindrance
delocalisation
rotamer
how does steric hindrance affect rates and barriers
destabilises transition states and raises barriers
how does delocalisation affect rates and barriers
stabilises ground states and raises barriers
what is a rotamer
an isomer arising from a hindered single bond rotation
give examples of some rotamers
how can we detect rotamers
the protons are distinguishable in NMR
describe a cyclohexane ring flip
the result of a bond rotation and any substituent that is axial becomes equatorial and vice versa
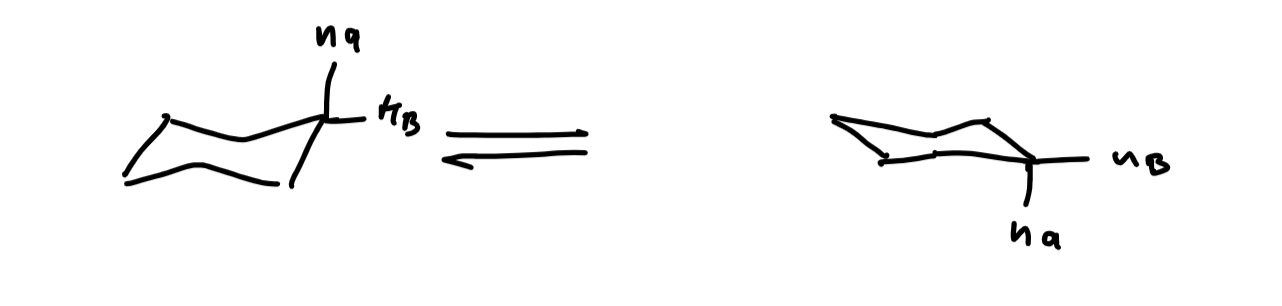
describe the conformational energy changes during the inversion of cyclohexane
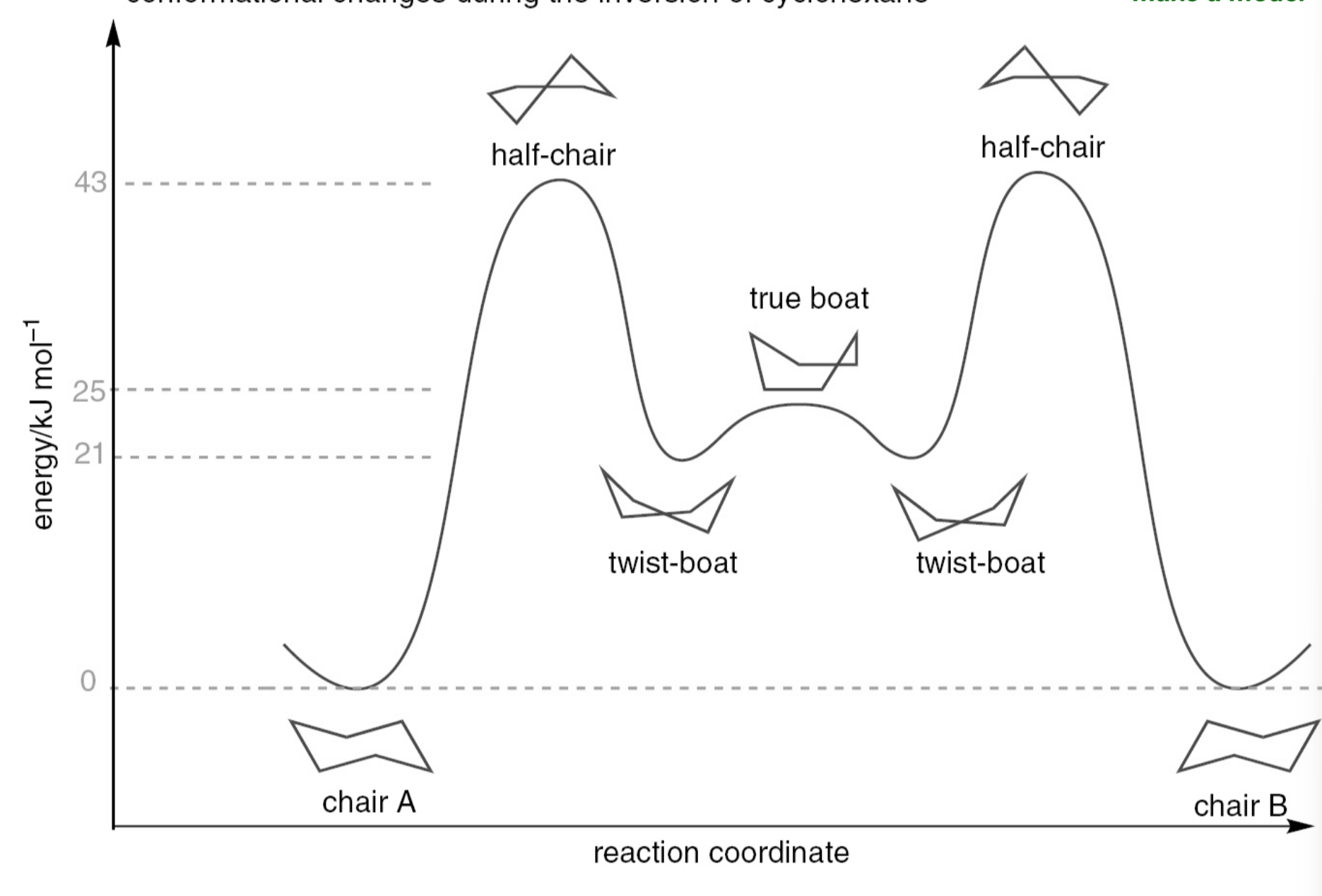
which conformer of cyclohexane is more thermodynamically stable
the conformer with the substituent axial is higher in energy in almost all cases meaning there will be less of this form present at equilibrium
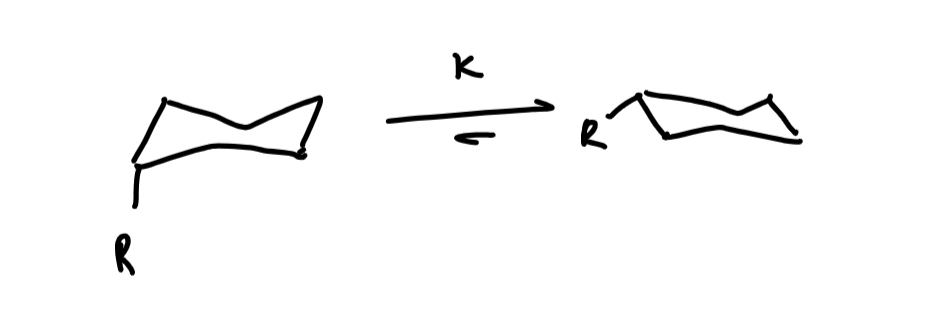
what is a conformationally locking group
sterically demanding groups
what is the anomeric effect
a notable example where axial substitution is favoured over equatorial in general when bearing an electronegative atom in the 2 position axial substitution is preferred
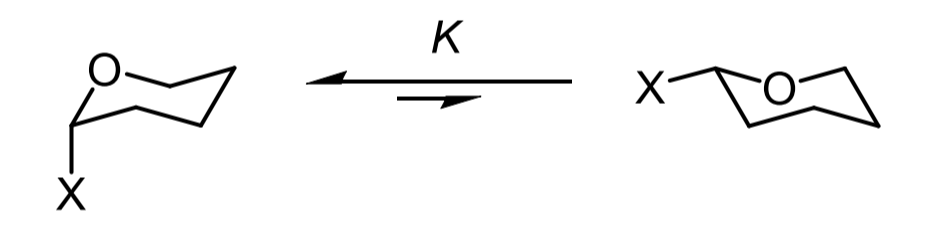
why does the anomeric effect occur
the axial lone pair can donate int the sigma star orbital of the axial substituent but if it where equatorial the lone pairs are incorrectly aligned for overlap

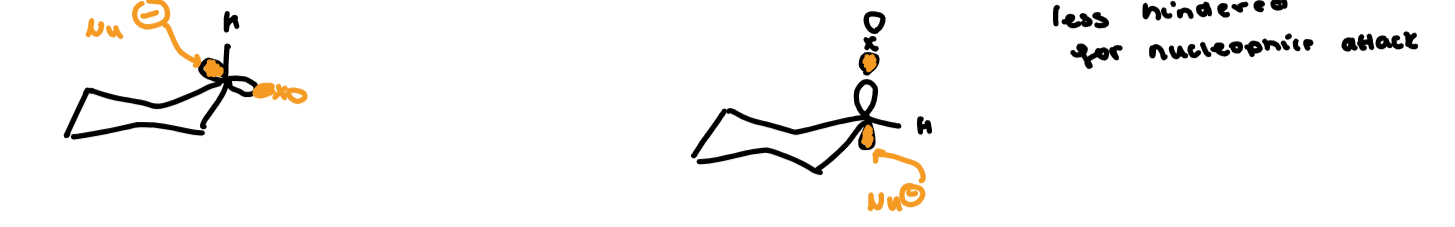
what conformation is preferred in an SN2 reaction
substitution is 31 times faster with an axial leaving group because it is less hindered for nucleophile attack
describe the elimination reaction of
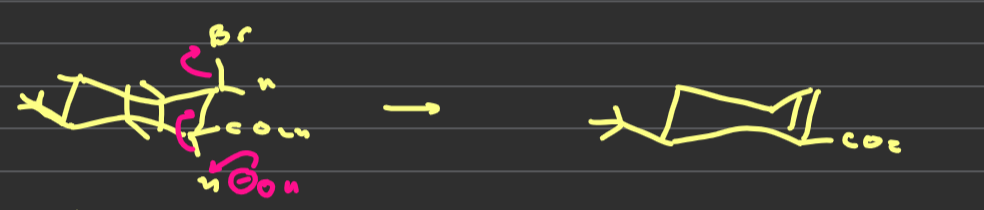
describe the elimination reaction of

how do the elimination reactions of these two molecules differ
in the trans conformation both groups are eliminated because they are anti periplanar to one another whereas in the cis only bromine leaves
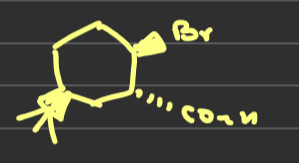
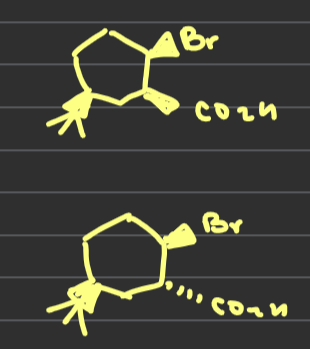
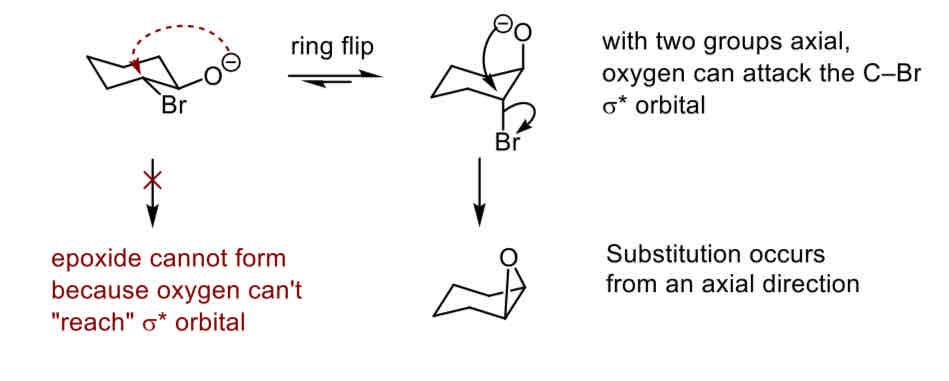
What conformation is required for the ring closing epoxide reaction and why
When both groups are axial the oxygen can attack the C-Br sigma* orbital and substitution occurs from an axial direction whereas when both groups are equatorial the epoxide cannot form because the oxygen can’t reach the sigma* orbitals so The minor conformer is the reactive conformer
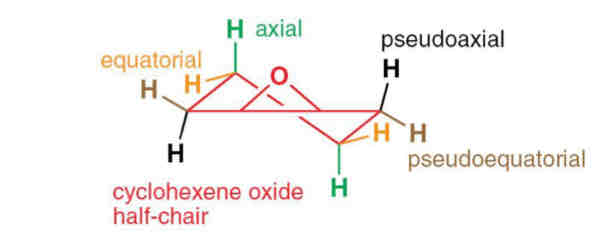
Describe the conformation of the cyclohexane epoxide
The distortion due to the three membered ring changes the orientation of the hydrogen atoms on the sp3 carbons bonded to it - pseuodaxial and pseuodoequitorial
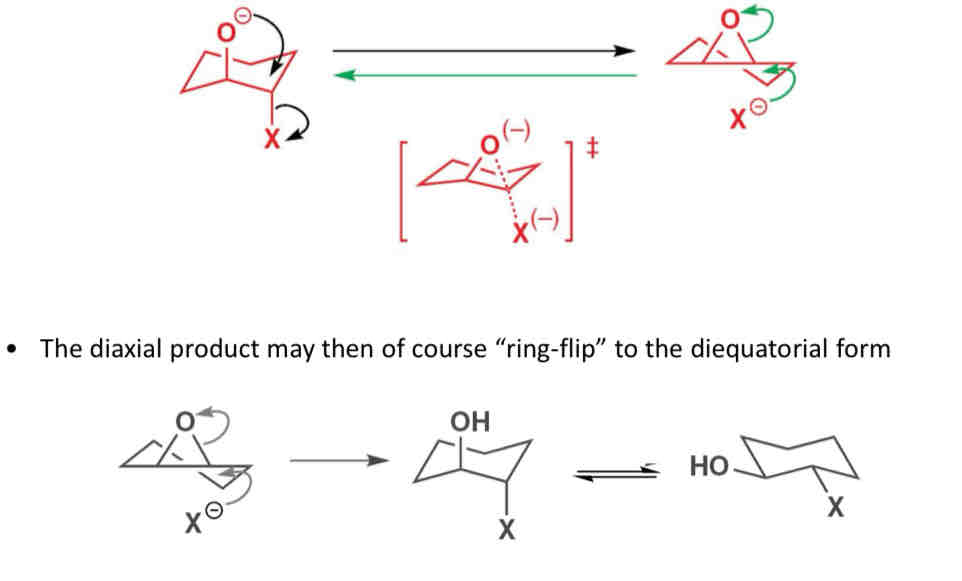
What is the principle of microscopic reversibility
If only a diaxial starting material can close to an epoxide a cyclohexane epoxide can only open to a diaxial product since the lowest energy transition state for a ring opening and losing must be the same
Describe the mechanism for the principle of microscopic reversibility
Describe regiochemical control in epoxides
If a bulky substituent (conformational lock) is present, ring flipping is unfavourable and the diaxial product remains diaxial
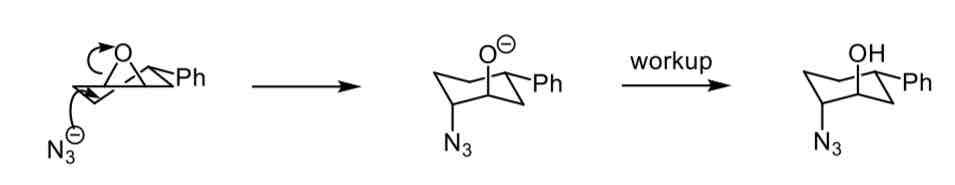
Describe the mechanism for opening an epoxide ring with a conformational lock present
The phenyl group fixes the conformation, it stays equatorial and the nucleophile attacks the epoxide at the position shown to ring open to a diaxially substituted chair conformation
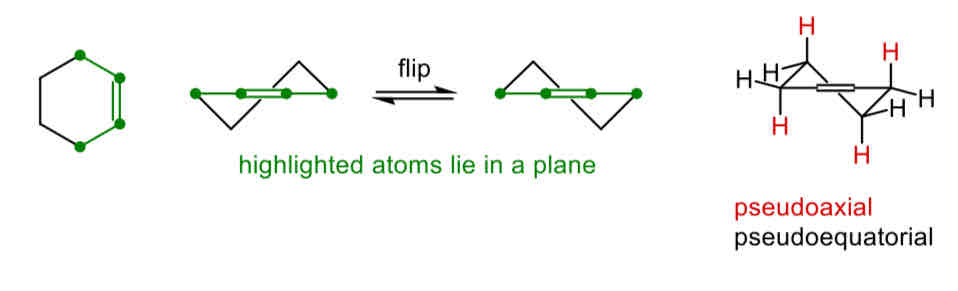
Describe the structure and reactivity of cyclohexene
With two sp2 carbons it adopts a half chair or flattened chair conformation, which is less stable than a chair so a process that renders them a hair is likely to be favoured Only axial attack is possible
Describe electrophilic attack on 5 membered cyclic alkenes
When there are two or more sp3 carbons in a 5-membered ring the ring is flatter still and can give rise to stereoselective reactions because the cyclopentene has two faces. There is preferential attack on the less hindered face
Describe the epoxidation of an r-cyclopentene with m-CPBA
This is a diastereoselective reactions because attack occurs preferentially on the less hindered face
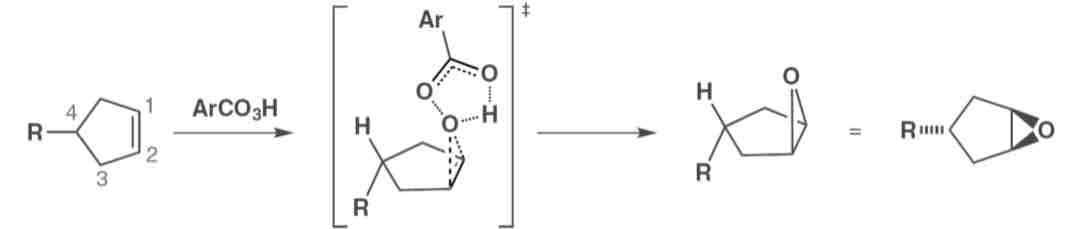
Describe the attack in a cyclohexene
Only axial attack is possible due to the orientation of the p-orbitals
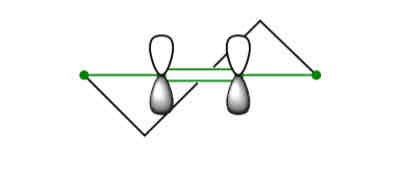
describe angle strain in cyclopropane
has the greatest angle strain by far
describe ring strain in cyclobutane
highly strained and adopts a puckered conformation
describe ring strain in cyclopentane
bond angles close to 109.5 and the ring distorts to reduce eclipsing C-H bonds, increasing angle strain
the minimum energy conformation is a result of balancing thse
describe ring strain in cyclohexane
these are essentially strain free and there are eclipsing interactions in boat conformer
describe ring strain in medium rings
ring size is 8-13 and puckering reduces ring strain
transannular (across ring interactions) strain makes medium rings hard to form however, once medium rings are formed transannular reactions are common
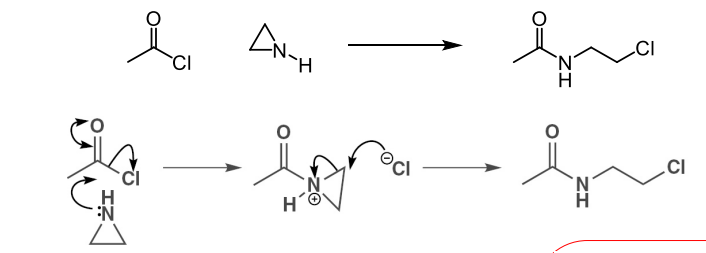
describe how ring strain promotes ring opening reactions with acyl aziridine
describe the general rate of reactivity for different ring sizes
5>6>3>7>4>8-10
forming small rings introduces ring strain leading to raised energy of transition state
proximity of reacting termini must also be important
describe the role of entropy in ring closing reactions
for ring sizes 5-7 there are many possible conformations and there is a large entropy as only one can react and there is a small change in enthalpy because there is no ring strain
for 3-4 rings there are few possible conformations and there is a small entropy because the reactive conformation is only one of a few
the ring strain also means a large enthalpy
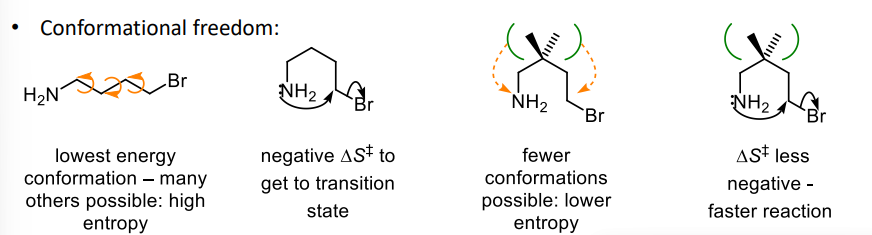
what is the gem-dimethyl effect
having geminal dimethyl groups lowers the conformational freedom which lowers the entropy so DS is less negative and the reaction is faster
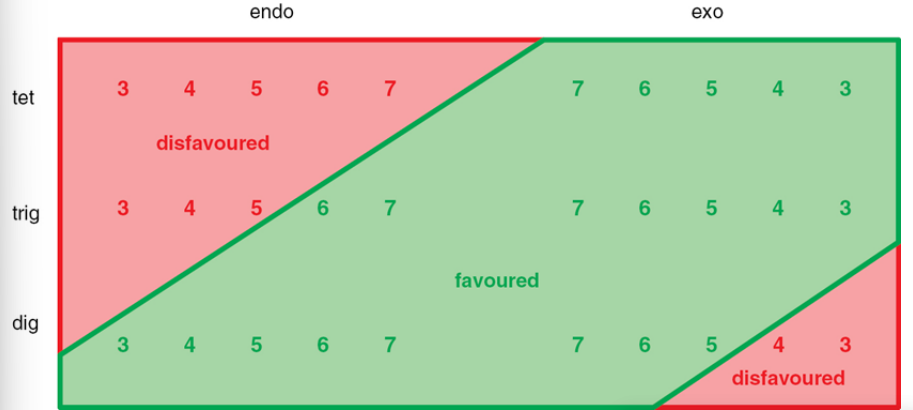
how can cyclization reactions be classified
the ring size being formed
whether the bond breaking in the ring closing step is inside (endo) or outside (exo) the new ring which is being formed
whether the electrophile is an sp3 (tetrahedral) sp2 (trigonal) or sp (digonal) centre
which exo cyclization’s are favoured
exo-trig and exo-tet cyclizations are favoured

what do endo trig reactions depend upon
they depend upon the ring size being formed
5-endo-trig is disfavoured however 5-exo-trig is a favourable process
it is disfavoured because there is a poor alignment of the lone pair and pi* orbital or it is too remote
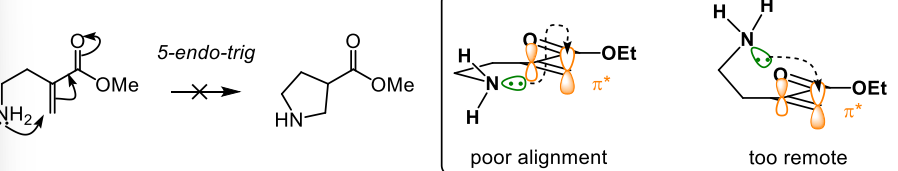
what endo-trig reactions are favoured
6-endo-tring becaue the chain is now long enough to allow efficient overlap
which cyclizations are most preferable
endo-dig cyclizations
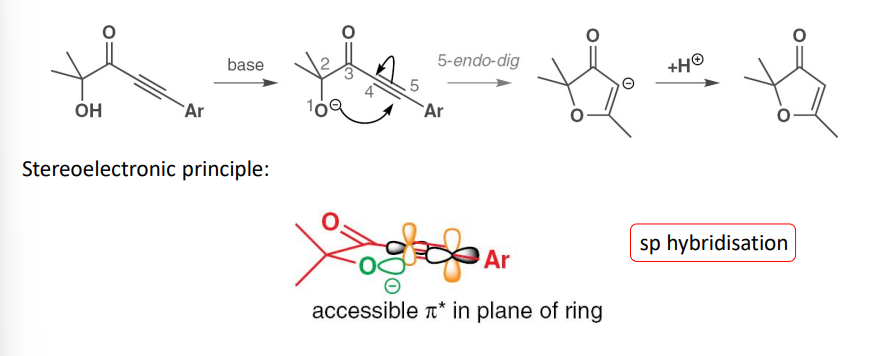
describe microscopic reversibility in terms of Baldwins rules
transition state is the same for the reverse process of ring opening
so 5-endo-trig is difavoured either way becuase reacting pi and sigma* orbitals are orthogonal and cannot interact
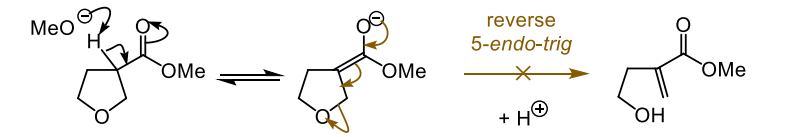
what are the guidelines which can be used to predict which ring closures will work
What are the exceptions of the Baldwin rules
Enolate cyclizations
Cyclizations involving cations
Reactions with second row heteroatom nucleophiles
Concerted process
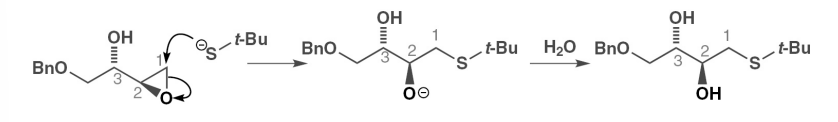
what is neighbouring group participation
the presence of a group/functionality in a molecule that is remote from a reacting centre having an effect on the rate and hence the outcome of a situation
define anchimeric assistance
neighbouring group participation
describe how a neighbouring group could participate via a 3 membered intermediate
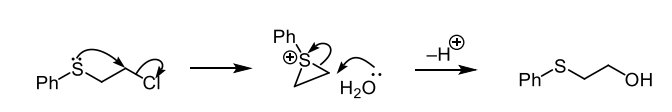
why may this reaction have a retention of stereochemistry
they are anti with respect to the double bond and the reaction occurs via a carbocation intermediate
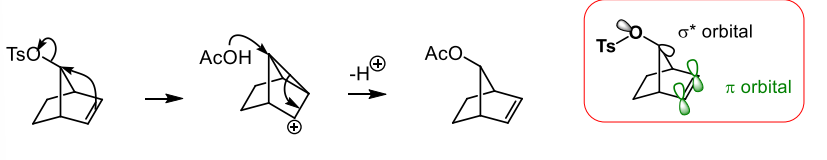
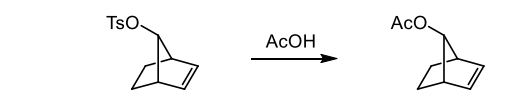
what does the presence of a single enantiomer in a reaction usually indicate
it rules out simple Sn1 and Sn2 reactions which suggests neighbouring group participation via a delocalised intermediate (mechanism which is similar to EAS)
or 2 Sn2 type reactions occurred ( inversion and then inversion again back to the same stereochemistry)
describe the mechanism of neighbouring groups migration
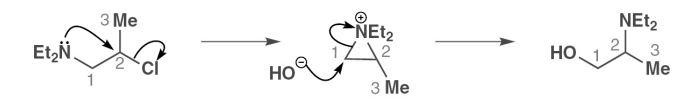
When does a net molecular rearrangement in a molecule occur
when there is both a good nucleophile and leaving group on the chain
when the intramolecular ring closing is Baldwin favoured
when the ring is a good electrophile (for example has a quaternary nitrogen)
when might an epoxide ring opening lead to movement of OH group onto a different carbon
in the presence of a strong nucleophile it will undergo ring opening
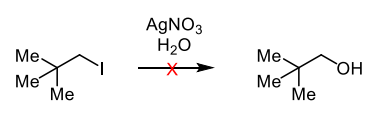
why are neopentyl halides generally poor substrates for nucleophilic substitution reactions
It is because they are too hindered for Sn2 and Sn1 would have to proceed via an unstable primary carbocation
describe the mechanism for how a neopentyl halide may undergo a nucleophilic substitution
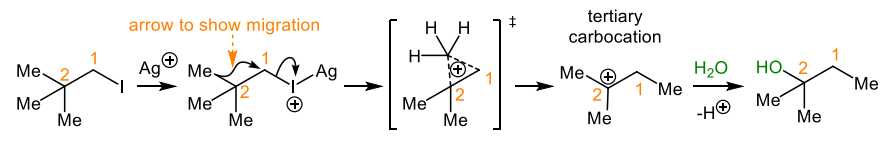
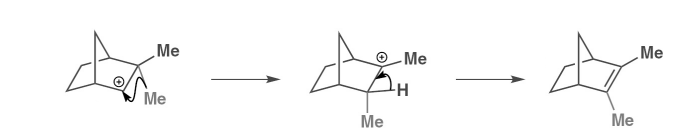
give an orbital description of the carbocation rearrangements
driving force is the formation of a tertiary carbocation
the alkyl migrations occur in the direction of the more stable carbenium ion
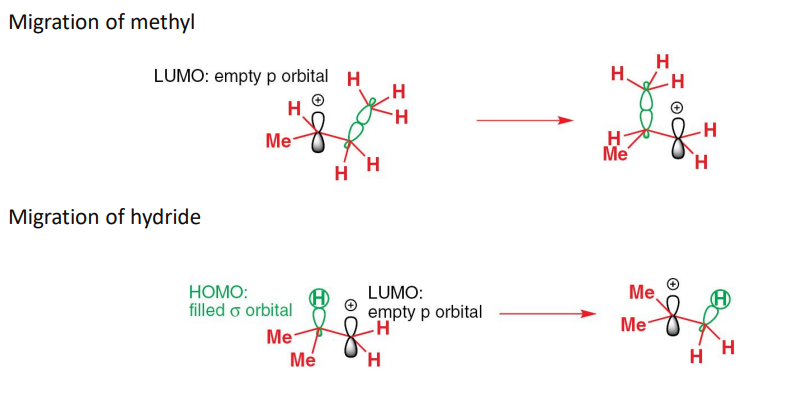
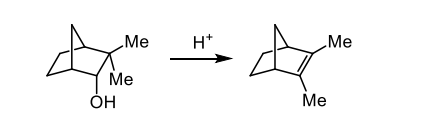
describe the elimination reaction
the mechanism is a 1,2-alkyl shift followed by an E1 elimination
what is Bredts rule
generally alkenes do not form at bridgeheads
what is a pinacol type reaction
if the product cation is stabilised by an adjacent -OH group it is called a pinacol rearrangement
describe the mechanism of a pinacol rearrangement
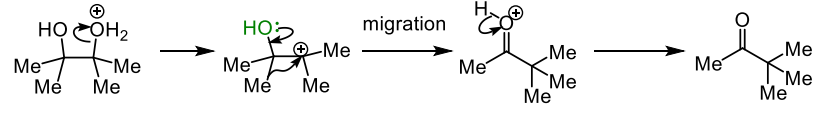
what is a semipinacol rearrangement
when the leaving group is other than water such as OTs
when is rearrangement common in rings
during ring expansion and contraction
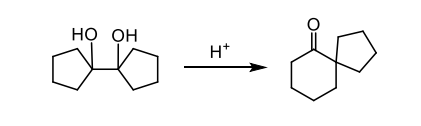
give the mechanism of a ring expansion via pinacol rearrangement
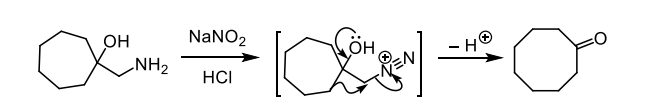
how do we form a diazonium salt
amine + NaNO2 and HCl
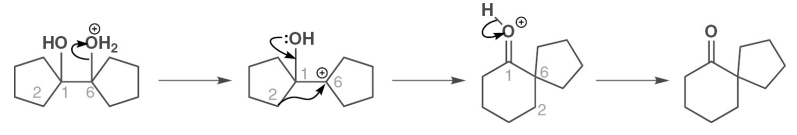
describe a semipinacol rearrangement on a ring with a diazonium salt
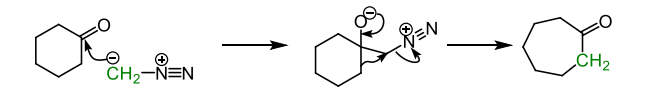
describe how else a ketone ring expansion without an amine may occur
treating the ketone with a diazomethane is a convenient alternative for ring expansion
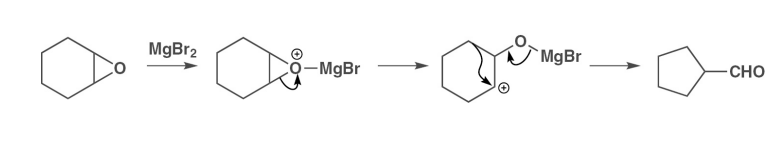
what is the mechanism for a pinacol epoxide ring opening into an aldehyde
treatment with a grignard reagent
what is the Beckmann rearrangement - How do we add a nitrogen to a cycolhexanone ring
the rearrangement of an oxime to an amide upon treatment with acid
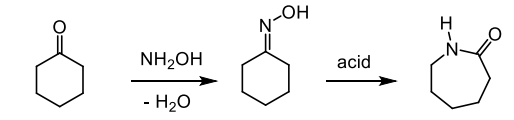
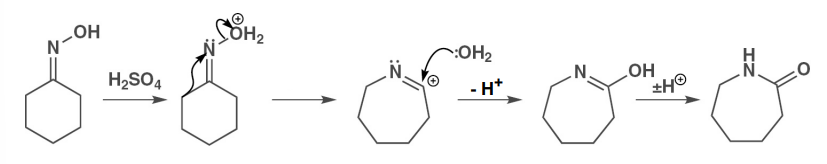
give the mechanism of the rearrangement of an axime to an amide
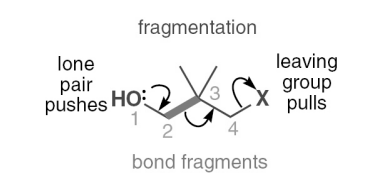
what is the general mechanism for fragmentation
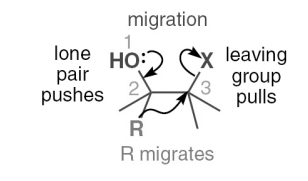
what is the general mechanism for migration
what is the basic structure of an amino acid
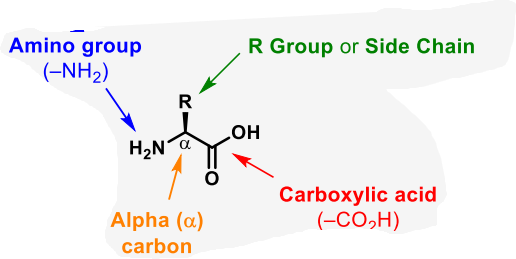
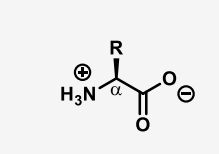
define zwitterion
a molecule that has separate negatively and positively charged group
how do we calculate the IEP
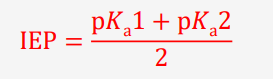
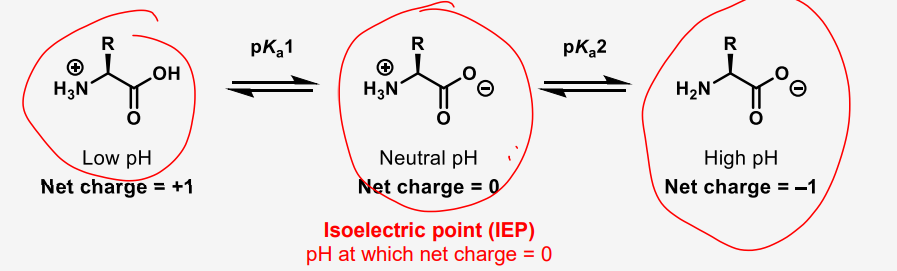
define IEP
the average of the two pKa values surrounding the structure with net 0 charge
describe what the resonance states of a simple amino acid look like
which amino acids have disfavoured ionisation states at physiological pH (7.4)
serine, threonine, cysteine, tyrosine, histidine
which amino acids has favoured ionisation states at physiological pH (7.4)
arginine, lysine, aspartic acid, glutamic acid
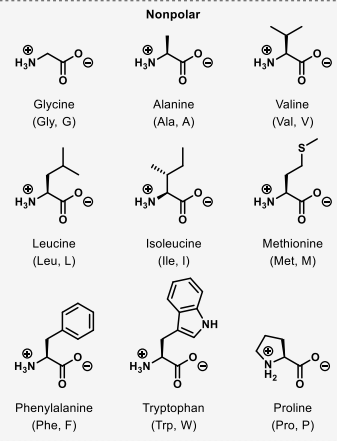
which amino acids are non-polar
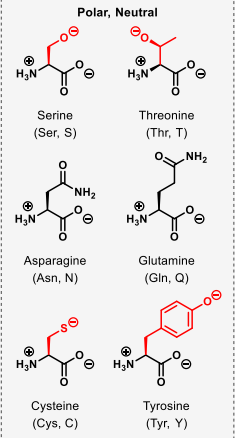
which amino acids are polar and neutral
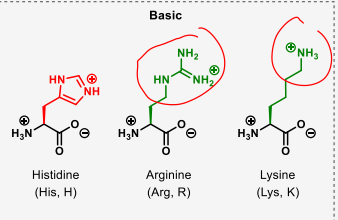
which amino acids are basic
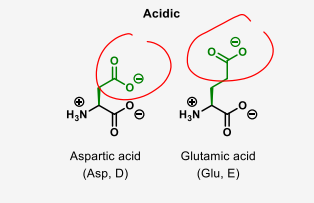
which amino acids are acidic
what type of interactions do amino acid side chains undergo
hydrogen bonding, van der Waals, disulfide bridges
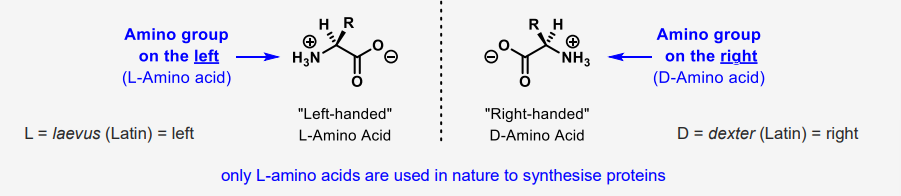
describe the chirality in an amino acid
what are the 4 types of amino acid synthesis
Strecker
Nucleophilic substitution of alpha-halocarboxylic acids
reductive amination
enantioselective biosynthesis
describe the Strecker amino acid synthesis
carbonyl—> nitrile—> amino acid
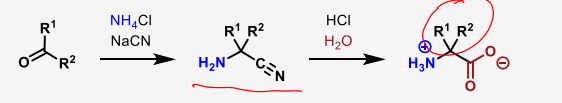
describe the nucleophilic substitution of alpha-halocarboxylic acids to form amino acids
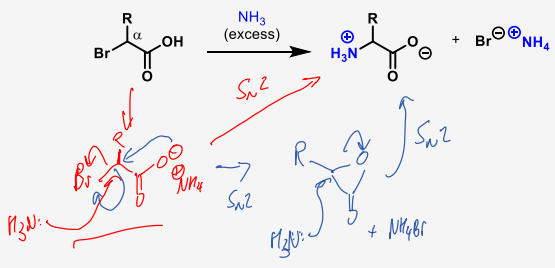
which SN2 reaction is preferred when forming an amino acid
SN2 reactions are very fast when adjacent to pi systems especially carbonyls due to stabilisation of the transition state, so there will not be a ring opening and closing reaction.
What can affect the rate if the SN2 formation of amino acids
it can slow for sterically hindered electrophiles
What is a requirement when forming an amino acid via SN2
excess ammonia is needed to prevent overalkylation
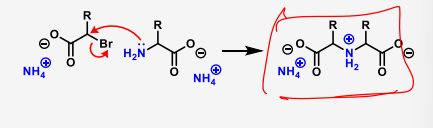
describe the chemical synthesis of amino acids using reductive amination
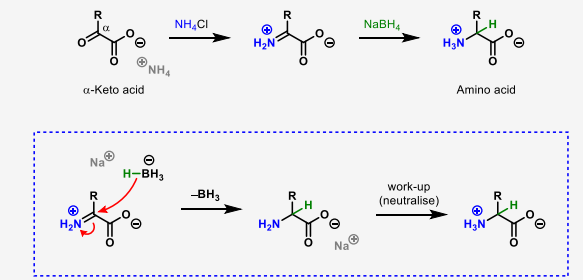
describe the enantioselective biosynthesis of amino acids
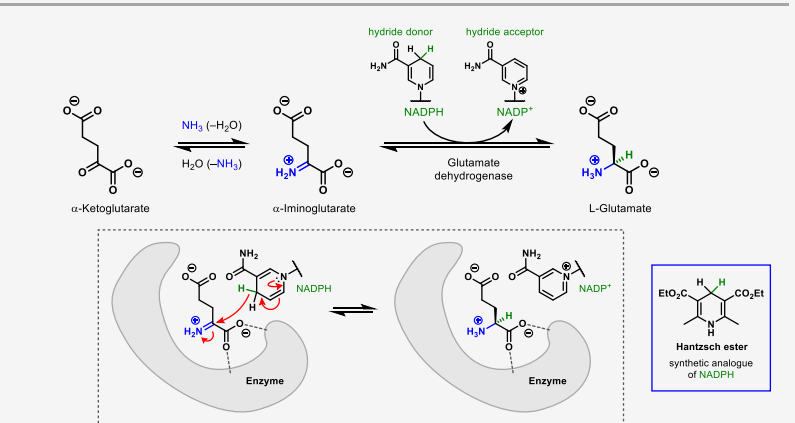
how do you know which synthetic pathway to use for amino acid synthesis
strecker synthesis for a doubly substituted ketone
nucleophilic substitution when there is a carboxylic acid adjacent to a leaving group
reductive amination for a double carbonyl compound
what is the basic structure of an amino acid
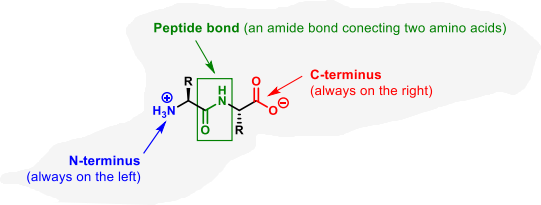
why do most peptides exist in the trans form?
because the cis peptide bond leads to steric clash which is strongly disfavoured
why are protecting groups required in amino synthesis
to ensure that the order of amino acids are correct by using amino or carboxyl protecting groups
what are the 3 amino protecting groups
carboxybenzyl- CBz
9-fluorenylmethoxycarbonyl - Fmoc
tert-butoxycarbonyl - Boc
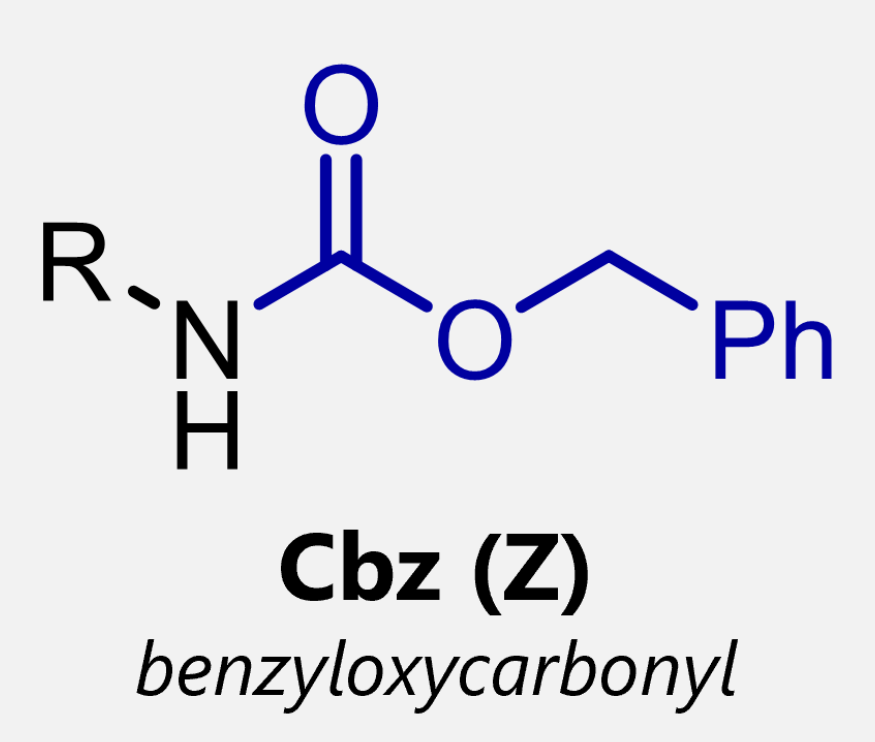
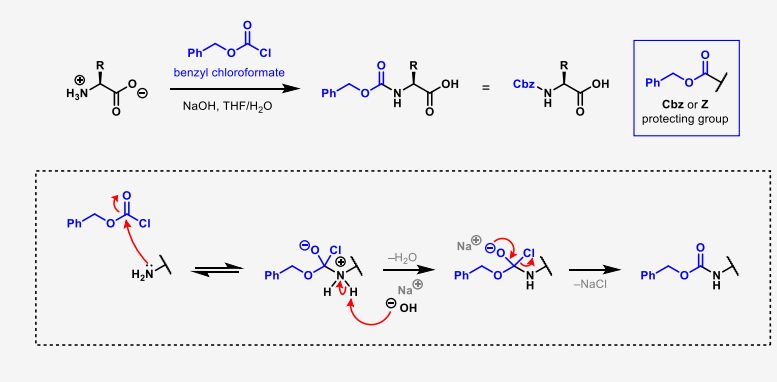
describe how to add carboxybenzyl as an amino protecting group
NaOH, THF H2O