chem 2 lab concepts
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
74 Terms
A
mass
Z
protons
alpha decay
-4 mass (A); -2 protons (Z)
beta decay/beta emission
+0 mass (A); +1 protons (Z)
position emission & electron capture
+0 mass (A); -1 protons (Z)
gamma decay
+0 mass (A); +0 protons (Z)
wut stops alpha
sheet of paper
wut stops beta
sheet of metal
wut stops gamma
thick concrete
zero order rxn equation
[A]t = -kt + [A]0
first order rxn equation
ln[A]t = -kt + ln[A]0
second order rxn equation
1/[A]t= -kt + 1/[A]0
[A]0
initial [ ]
t
time elapsed
[A]t
[ ] of the react @ any time
k
rate constant
zero order half life
t1/2 = [A]0 / 2k
first order half life
t1/2 = ln(2) / k
second order half life
t1/2 = 1 / k[A]0
% error
( |actual - theoretical| / theoretical ) * 100
on calibration curve
[ ] is the independent variable (x axis)
micro mL to mL
1000 micro mL = 1 mL
1 × 10^-6 L
1 micro mL
1 × 10^-6 M
1 micro M
Ka
acid dissociation constant
high Ka
strong acid; fully dissociates in water
low Ka
weak acid; doesn’t fully dissociates in water
low Ksp
insoluble
high Ksp
soluble
Ksp
solubility product constant
in cell notation
anode on the left & cathode on the right
FATCAT
from anode to cathode
Q in Nerst equation
[dilute solution] / [concentrated solution]
strong field ligand
low spin; pair first
weak field ligand
high spin; fill orbitals (unpaired)
low delta octahedral
longer lambda absorbed
high delta octahedral
shorter lambda absorbed
violet
400-450 nm
blue
450-500 nm
green
500-550 nm
yellow
550-600 nm
orange
600-650 nm
red
650-700 nm
y in y=mx+b
absorbance
x in y=mx+b
concentration
m in y=mx+b
constant
b in y=mx+b
y-intercept
all sodium salts
are soluble
neutral solution is formed
salts with both strong acid & strong base (cation & anion)
what is neutral for cations
G1 & G2 metals
what is neutral for anions
strong acid anions
if cation strong (base)
basic
if anion strong (acid)
acidic
y=mx+b for thermo
m:S & x:T
black electrode
negative
red electrode
positive
high #E
keep; cathode
oxi agent
accepts e-; reduced e- on right
red agent
donated e-; oxidized; e- on left
coordination #
num. of atoms bonded to central atom
oxi #
degree of oxi/red → charge (?)

environmental hazard

toxic
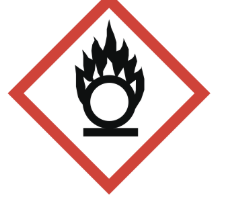
oxidizing

health hazard
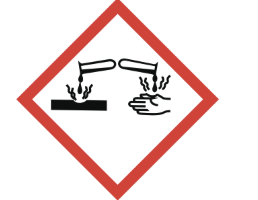
corrosive

flammable

harmful
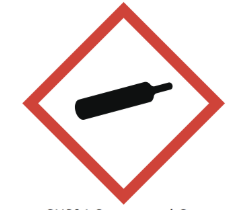
compressed gas
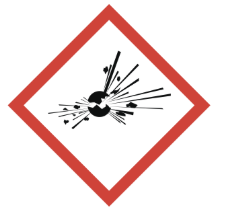
explosive
bidentate - 2
en, C2O4
polydenate - 6
EDTA,EDTA^4-
ploydentate - 3
dien
polydentate - 5
EDTA^3-