Week 6 - Mechanotransduction & ECM biomimicry
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
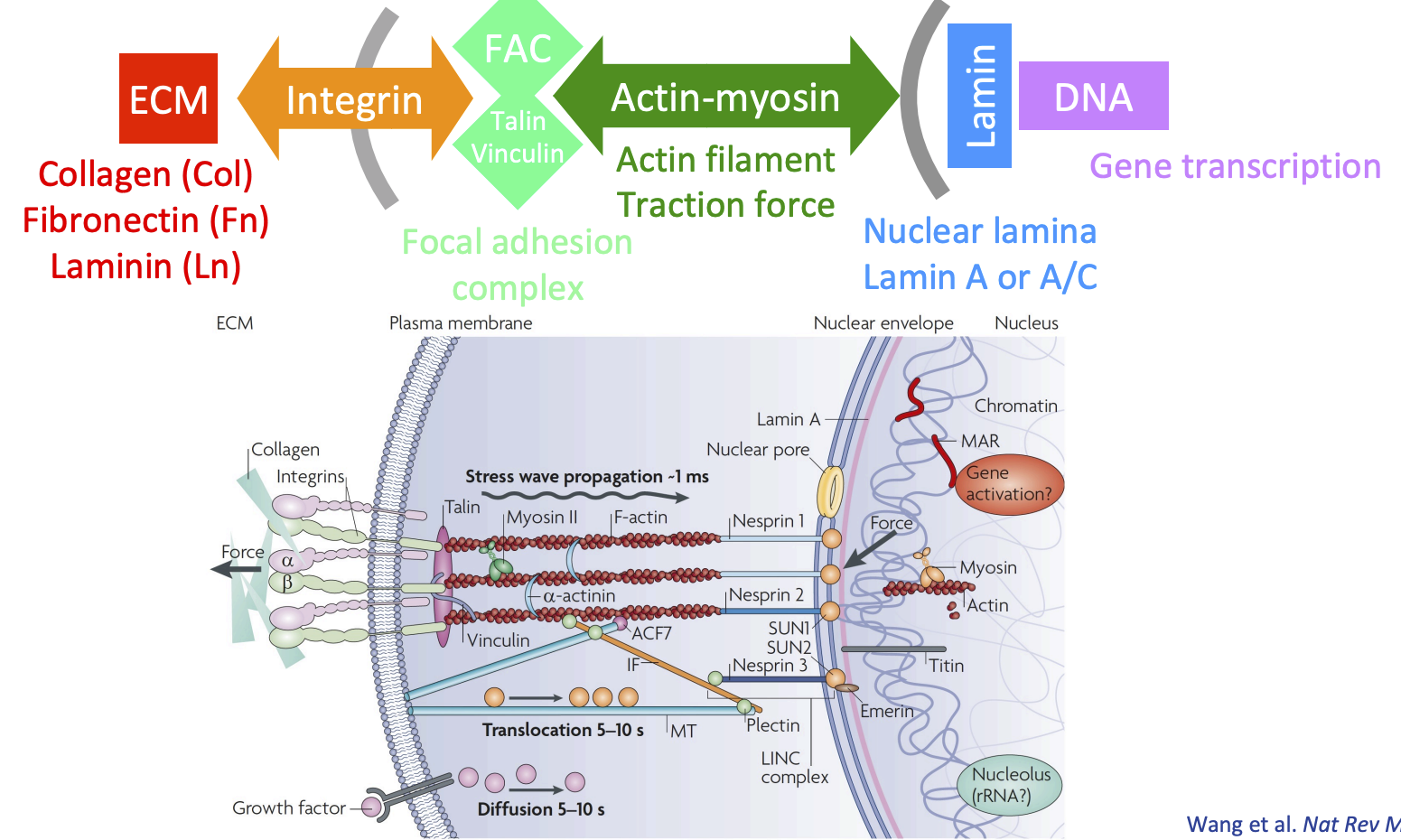
Integrin mediated mechanotransduction
Amino acid sequence in ECM recognised by integren
Beta subinit of integrin recruits adhesion proteins (talin/vinculin) = focal adhesion complex
Focal adhesion complex works with actin filaments to generate forces on nuclear lamina (lamin A or A/C)
= can push/pull (loosen/condense) DNA = affects gene transcription
ECM properties
size/shape
protein composition
stiffness
(affects integrin mediated mechanotransduction)
extracellular environment can influence traction force generation
Traction forces (actin-myosin)
Generated based on ECM stiffness
sliding action between actin filament and heads of non-muscle myosin = traction force generated
this movement of the myosin and actin filament allow the cell to move → much like the contraction of muscle
Myosin 2 (non-muscle) is important for cell adhesion and migration
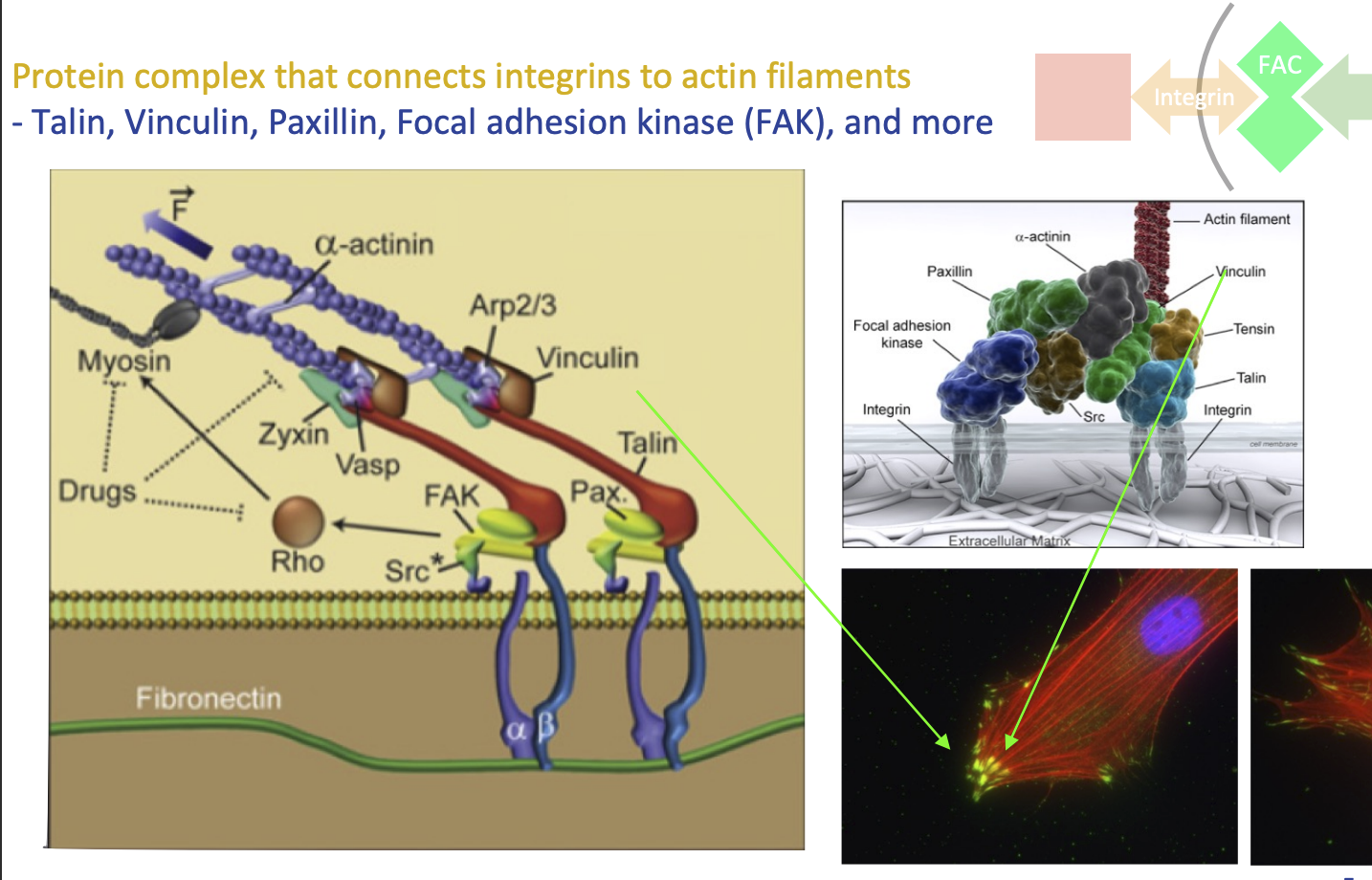
Focal adhesion complex
Protein complex that connects integrins to actin filaments
FAC proteins:
Talin
Vinculin
Paxillin
Focal adhesion kinase (FAK), and more
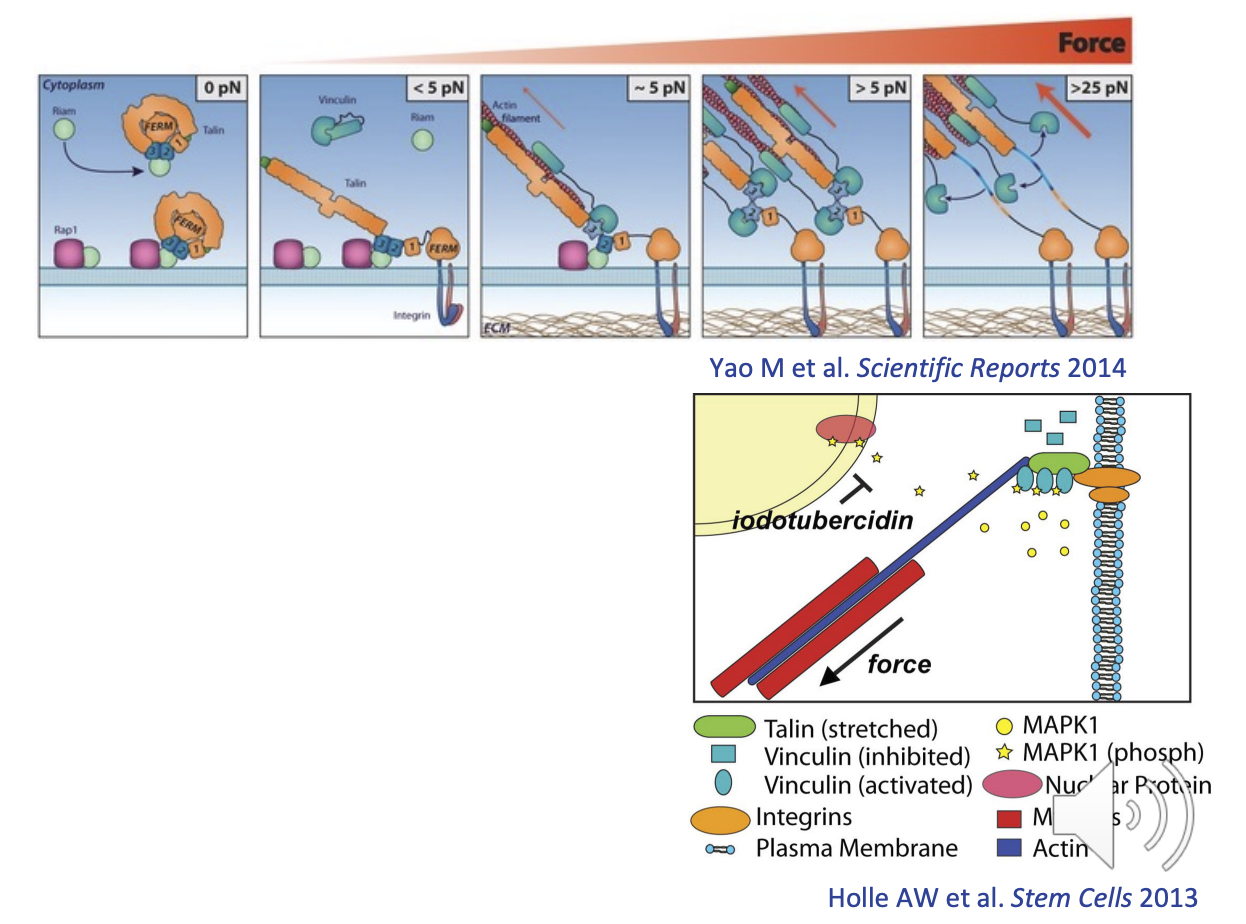
Talin-Vinculin binding sites & Mechanosensor properties
Talin & Vinculin can sense the strength of the tension due to their cryptic binding sites
their binding sites are exposed/activated when the molecule is stretched
Talin = up to 9 binding sites for vinculin
Vinculin = up to 3 for MAPK
concentration difference between bound and unbound vinculin can indicate the level of forces the cell is experiencing
unbound = less force
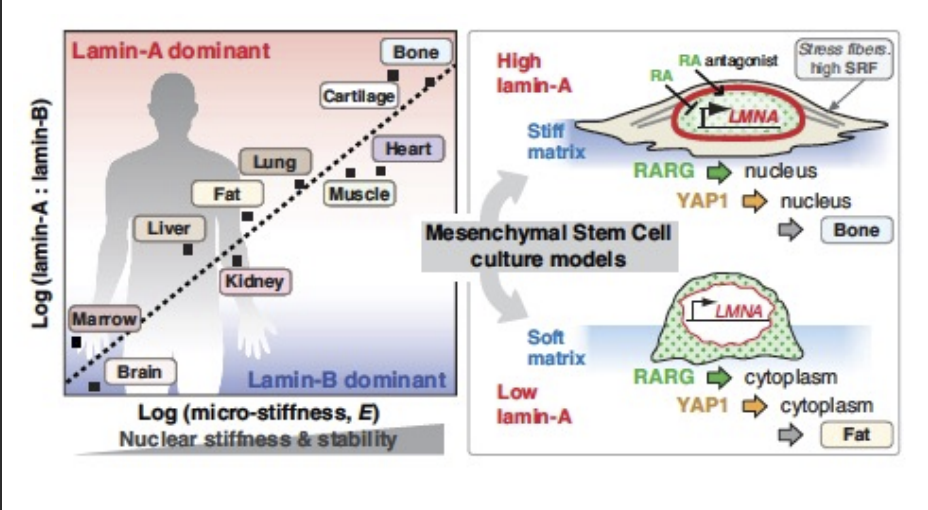
Nuclear lamina
built by Lamin A/B/C and intermediate filaments
higher conc. of Lamin A leads to greater stiffness
provides mechanical strength
external environment can stiffen the nuclear lamina
Lines the nucleus to protect DNA
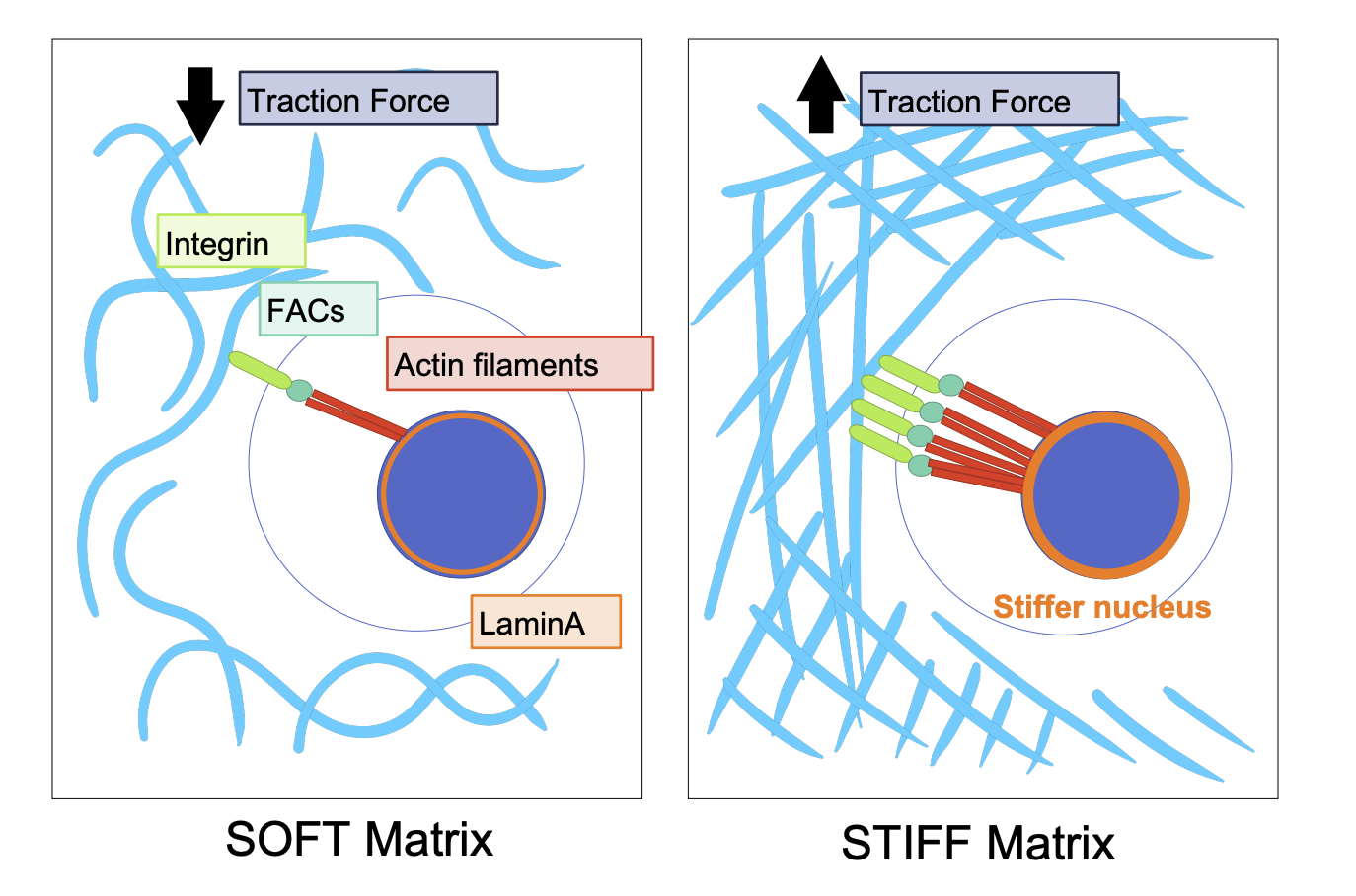
Stiff vs Soft ECM mechanotransduction
Soft = minimal integrins = minimal actin filaments = minimal traction force generated
Stiff = more traction force (opposite) = stiffer nucleus
YAP/TAX translocalisation
As stiff stiffness increases, expression of YAP/TAZ move from cytoplasm to nucleus
controls cell fate = differentiation
Larger traction force = translocalisation into nucleus = more transcription
cell can react differently → either going into sleep or more activity
MRTFa forms
Similar to YAP/TAZ it acts as a co-transcription factor
MRTFa - G-actin complex:
cytoplasmic (inactive)
Globular form
MRTFa without G-actin:
when cell stiffens, g-actin is released → MRTFa enters nucleus → acts as transcription regulator
comes out of nucleus to bind with g-actin when stiffness decreases
Mechano-memory
if cells are exposed to high stiffness for longer period of time →
sensitivity is changed
the effects of a mechanical stimulus persist long after the stimulus has been removed the mechanical memory of cells leads to lasting changes in behavior and function.
biomimicry process and challenge (3 steps)
Understand the normal conditions in healthy tissue → ECM in vitro
Mimic the disease → to study cell response
Cell death leaves scar tissues
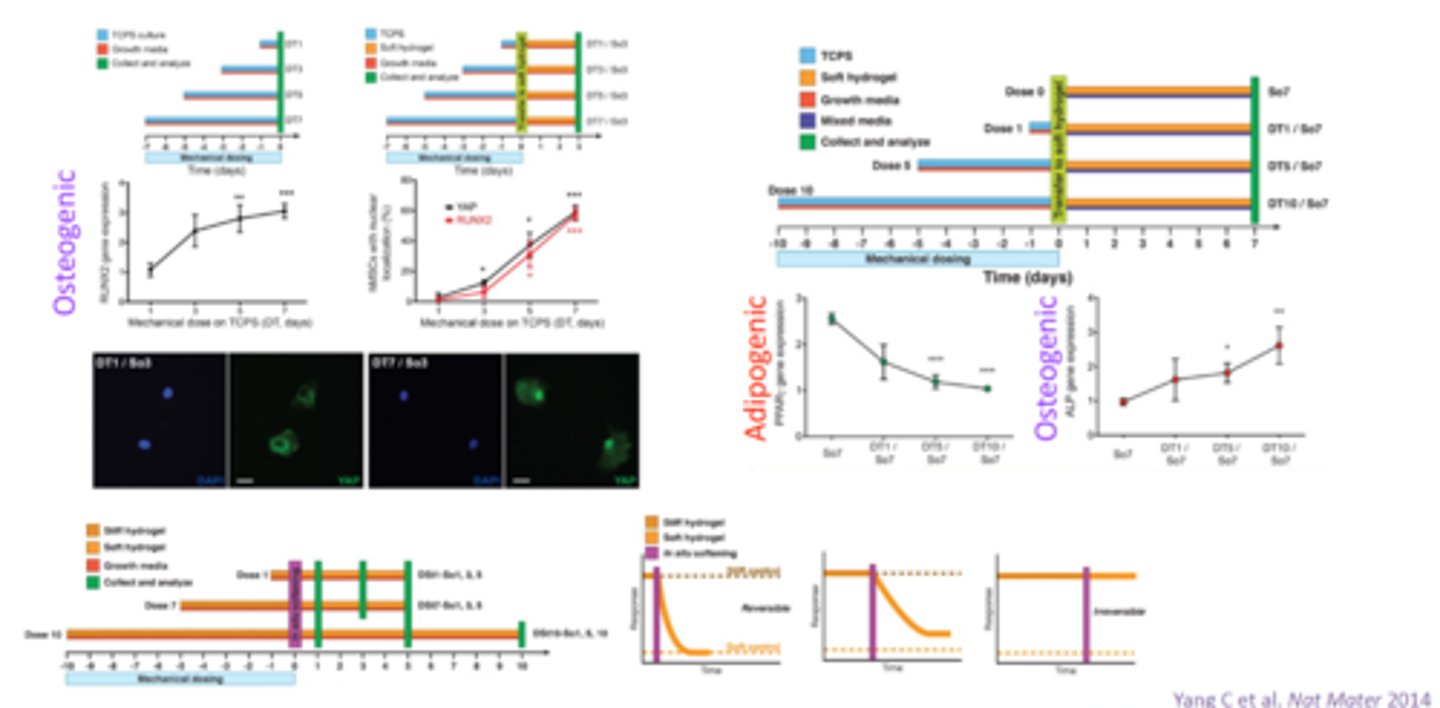
ECM stiffness and tissue regeneration
stiffness is an obstacle in disease for regeneration
Stiffness can change due to:
development stage
ageing
disease
Example (heart attack):
after heart attack, stiffness increases
causes stem cells to differentiate into bone (instead of cardiac muscle)
Hydrogel
water containing fibrous network
recapitulating few components of native tissue ECM
Stiffness controlled by
altering amount of polymer
and/or degree of crosslinking
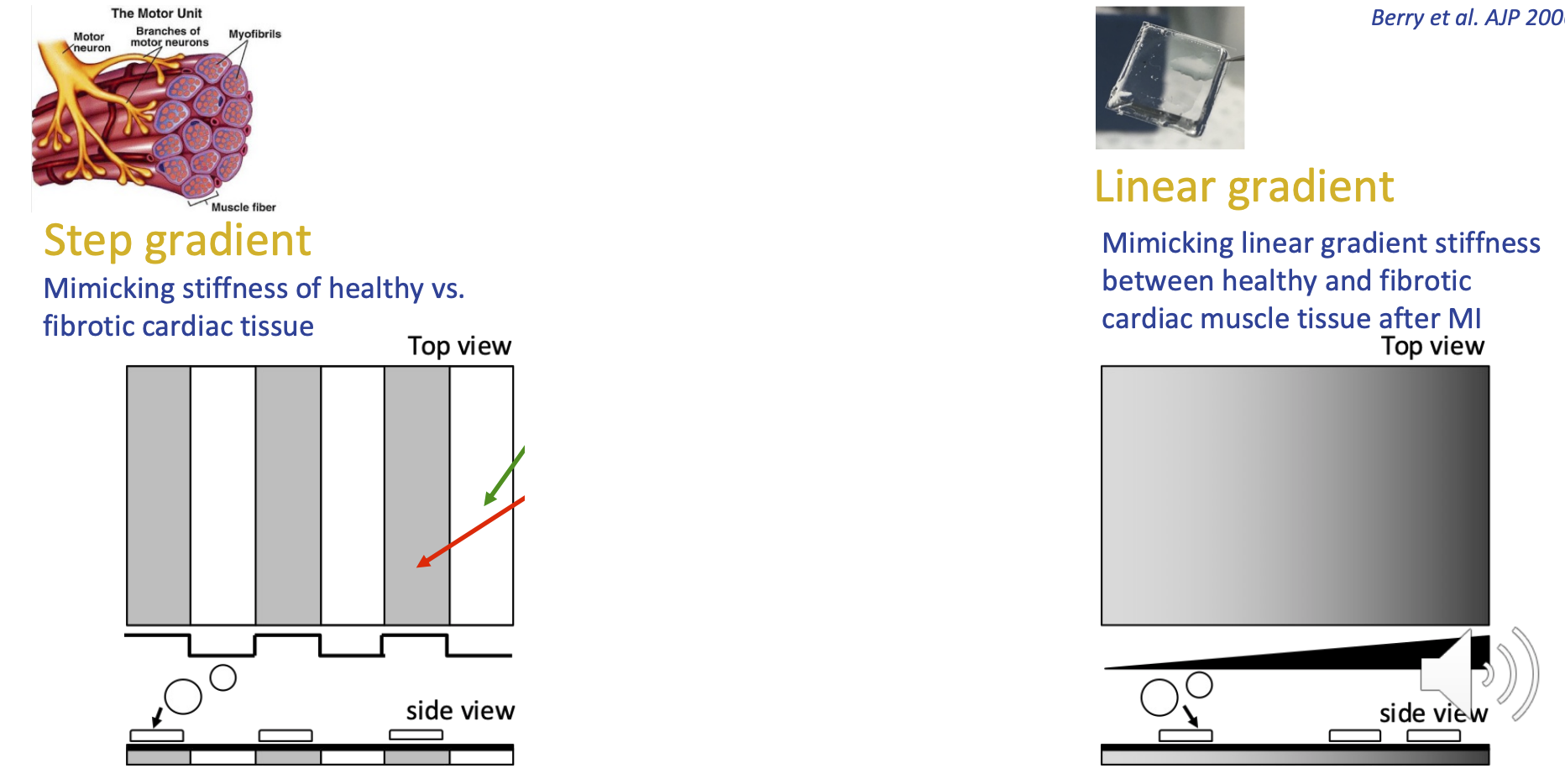
Mimicking ECM properties (stiffness)
Step gradient = mimicking healthy tissue vs fibrotic cardiac tissue
one strip of each
Linear gradient = mimicking linear gradient between healthy and fibrotic cardiac muscle tissue after MI
gradual transition from healthy to disease-like
Durotaxis
stiffness driven cell migration
In the ECM:
cells migrate to stiffer regions due to durotaxis
cells sense stiffness via various integrin pairs (during durotaxis)
Can cause larger traction force on one side of cell
Digital stiffness writing
hydrogel polymerisation initiated by photo/thermo-initiation using either energy from photon or heat
creating stiffens gel
digital stiffness writing uses infared laser to cause increased stiffness in gel
Decellularised ECM properties and pros/cons
Sits on top of linear gradient PA gel
take out the native tissue and remove the cells
Freeze dry
Rehydrate to form ECM like hydrogel
Pro = better biomimicry compared to using one/few ECM components
Cons = hard to pinpoint main contributor to cell behaviour
Microcontact printing
micro-sized stamps that create an adhesive ECM island
with specific shape and size to restrict/control cell shape/size
can be specialised to also have different stiffness
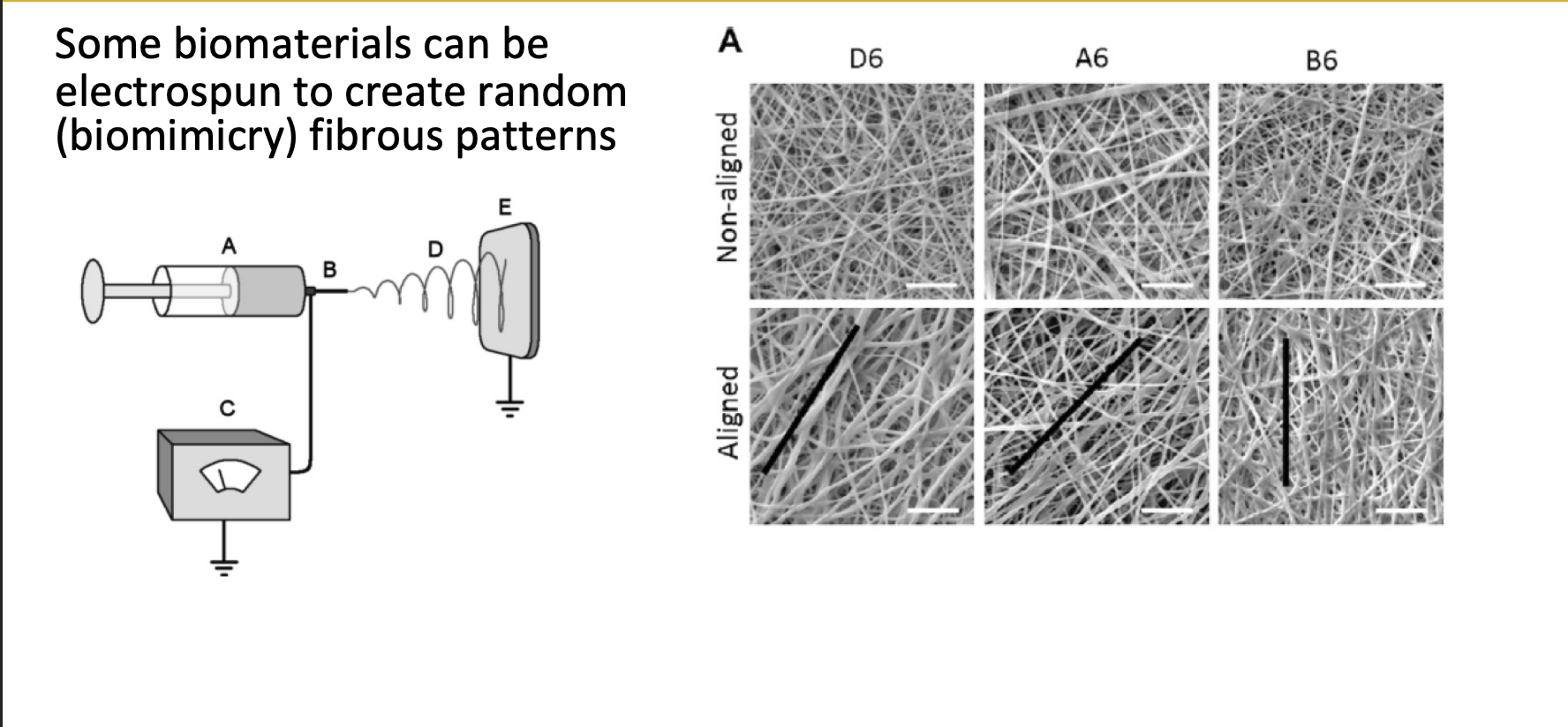
Electrospinning
biomaterials can be electrospun to create random fibrous patterns
mimic random arrangement of real tissue
3D bioprinting (biomimicry)
osteo-chondral interface can be mimicked by layer-by-layer 3D bioprinting
Chemically regulated biomaterials can...
alter stiffness + amino acid presentation
= control either/both stiffness and amount of integrin-binding ligands
= can examine cell mechanosensation