Rate of reaction
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
Describe an experiment to investigate the effects of changes in the surface area of a solid on the rate of a reaction, the effects of changes in the concentration of solutions on the rate of a reaction and the effects of changes in temperature on the rate of a reaction:
The rate of a chemical reaction can be measured either by how quickly reactants are used up or how quickly the products are formed.
The rate of reaction can be calculated using the following equation:

The units for rate of reaction will usually be grams per min (g/min)
An investigation of the reaction between marble chips and hydrochloric acid:

Marble chips, calcium carbonate (CaCO3) react with hydrochloric acid (HCl) to produce carbon dioxide gas. Calcium chloride solution is also formed.
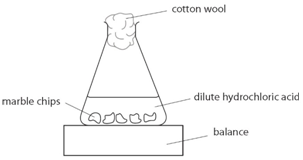
Using the apparatus shown, the change in carbon dioxide mass can be measured with time.
As the marble chips react with the acid, carbon dioxide is given off.
The purpose of the cotton wool is to allow carbon dioxide to escape but to stop any acid from spraying out.
The mass of carbon dioxide lost is measured at intervals, and a graph is plotted:
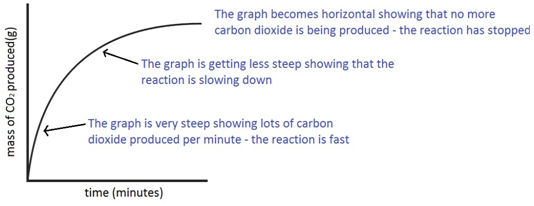
The experiment is repeated using the same mass of chips, but this time the chips are larger, i.e. have a smaller surface area.
Since the surface area is smaller, the rate of reaction is less.
Both sets of results are plotted on the same graph.
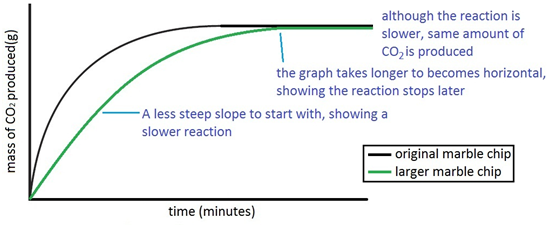
If instead the chips were smashed into powder (and again the same mass of chips used) the surface area would be much larger and so the rate of reaction would be ion higher (steeper line on the graph).
Experiment to investigate the effects of changes in the concentration of solutions on the rate of a reaction:
The experiment is again repeated using the exact same quantities of everything but this time with half the concentration of acid. The marble chips must however be in excess. The reaction with half the concentration of acid happens slower and produces half the amount of carbon dioxide.
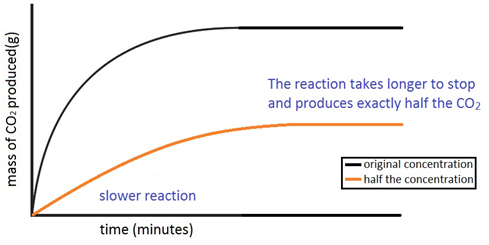
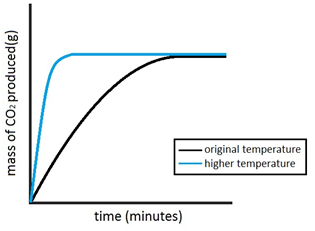
Experiment to investigate the effects of changes in temperature on the rate of a reaction:
The experiment is once again repeated using the exact same quantities of everything but this time at a higher temperature. The reaction with the higher temperature happens faster.
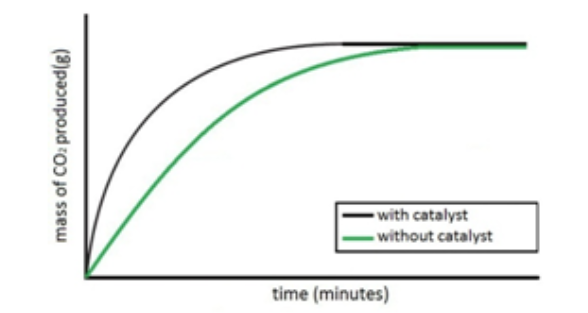
Describe an experiment to investigate the effects of the use of a catalyst on the rate of a reaction.
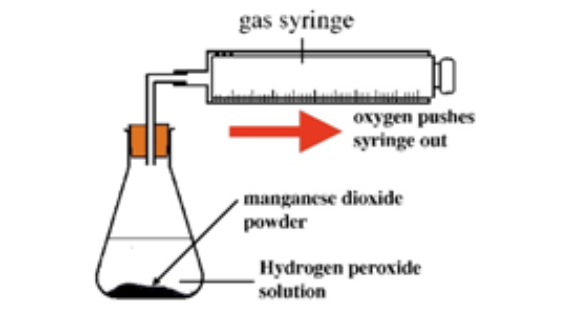
Experiment to investigate the effects of the use of a catalyst on the rate of a reaction:
Hydrogen peroxide naturally decomposes slowly producing water and oxygen gas.
Manganese (IV) oxide can be used as a catalyst to speed up the rate of reaction.
The rate of reaction can be measured by measuring the volume of oxygen produced at regular intervals using a gas syringe.
Both sets of results are plotted on the same graph.
Describe an experiment to investigate the reaction between varying concentrations of sodium thiosulfate and hydrochloric acid.
Experiment to investigate the reaction between varying concentrations of sodium thiosulfate and hydrochloric acid
Sodium thiosulfate (Na2S2O3) and hydrochloric acid (HCl) are both colourless solutions. They react to form a yellow precipitate of sulfur.
sodium thiosulfate + hydrochloric acid → sodium chloride + sulfur dioxide + sulfur + water
Na2S2O3(aq) + 2HCl(aq) → 2NaCl(aq) + SO2(g) + S(s) + H2O(l)
To investigate the effects of changes in concentration of sodium thiosulfate on the rate of a reaction, the conical flask is placed above a cross. The reaction mixture is observed from directly above and the time for a cross to disappear is measured. The cross disappears because a precipitate of sulfur is formed.
In order to change the concentration of sodium thiosulfate, the volumes of sodium thiosulfate and water are varied (see results table). However the total volume of solution must always be kept the same as to ensure that the depth of the solution remains constant.
In this reaction, sulfur dioxide gas (SO2), which is poisonous is produced therefore the experiment must be carried out in a well ventilated room.

The results are recorded in the table below and then plotted onto a graph.
Volume of Na2S2O3(aq (cm3) | Volume of water (cm3) | Concentration of Na2S2O3(aq) (mol/dm3) | Time taken for cross to disappear (s) | Rate of reaction (s-1) (1/time) |
50 | 0 | 0.10 | 45 | 0.0222 |
40 | 10 | 0.08 | 60 | 0.0167 |
30 | 20 | 0.06 | 80 | 0.0125 |
20 | 30 | 0.04 | 13 | 0.0769 |
10 | 40 | 0.02 | 255 | 0.0039 |
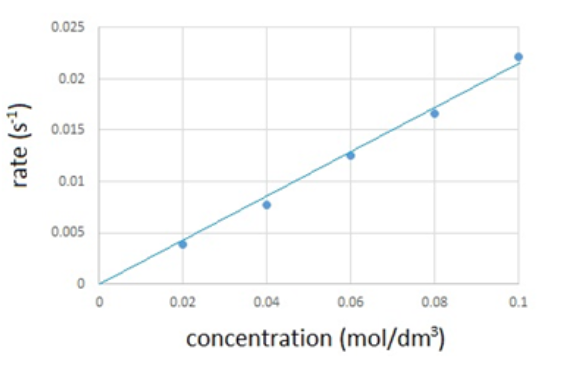
The graph shows that the rate of reaction is directly proportional to the concentration.
The experiment can also be repeated to show how temperature affects the rate of reaction.
In this experiment the concentration of sodium thiosulfate is kept constant but heated to range of different temperatures.
As a rough approximation, the rate of reaction doubles for every 10oC temperature rise.
What are the effects of changes in surface area of a solid, concentration of a solution, pressure of a gas and temperature on the rate of a reaction
Increasing the surface area of a solid:
more particles exposed
more successful collisions per unit time with energy greater or equal to the activation energy
increase the rate of a reaction
Increasing the concentration of a solution or pressure of a gas:
more particles in same space
more successful collisions per unit time with energy greater or equal to the activation energy
increase rate of reaction
Increasing the temperature:
particles have more kinetic energy
more frequent collisions
and a higher proportion of those collisions are successful because the collision energy is greater or equal to the activation energy
increase rate of reaction
What is a catalyst and how does it work?
A catalyst is a substance that increases the rate of a reaction, but is chemically unchanged at the end of the reaction.
A catalyst is not used up in a reaction.
A catalyst speeds up a reaction by providing an alternative pathway with lower activation energy.
How do you draw and explain reaction profile diagrams showing Δ H and activation energy?
Below is a diagram showing the reaction profile for the reaction of hydrogen with oxygen, which is EXOTHERMIC:
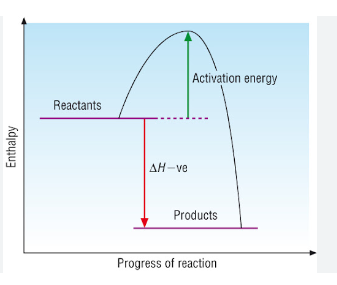
The activation energy is the minimum amount of energy required to start the reaction.
For an exothermic reaction, the products have less energy than the reactants. The difference between these energy levels is ΔH.
For an exothermic reaction, more energy is released when bonds are formed than taken in when bonds are broken.
Below is a diagram showing the reaction profile for the thermal decomposition of calcium carbonate, which is ENDOTHERMIC:
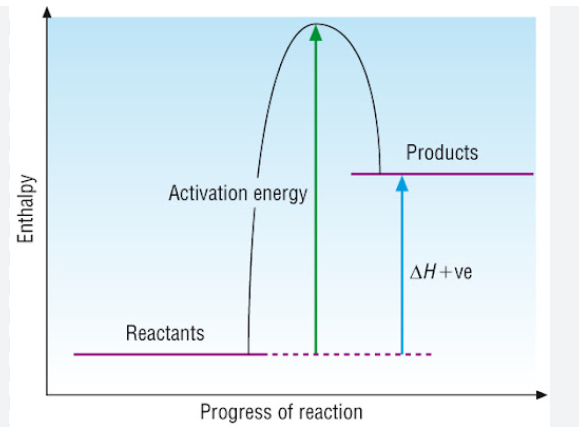
The activation energy is the minimum amount of energy required to start the reaction.
For an endothermic reaction, the products have more energy than the reactants. The difference between these energy levels is ΔH.
For an endothermic reaction, more energy is taken in to break bonds than is released when new bonds are formed.
Describe an experiment to measure the effect of a catalyst on the rate of reaction
Practical: Effect of catalysts on rate of reaction
Aim:
To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide
Diagram:
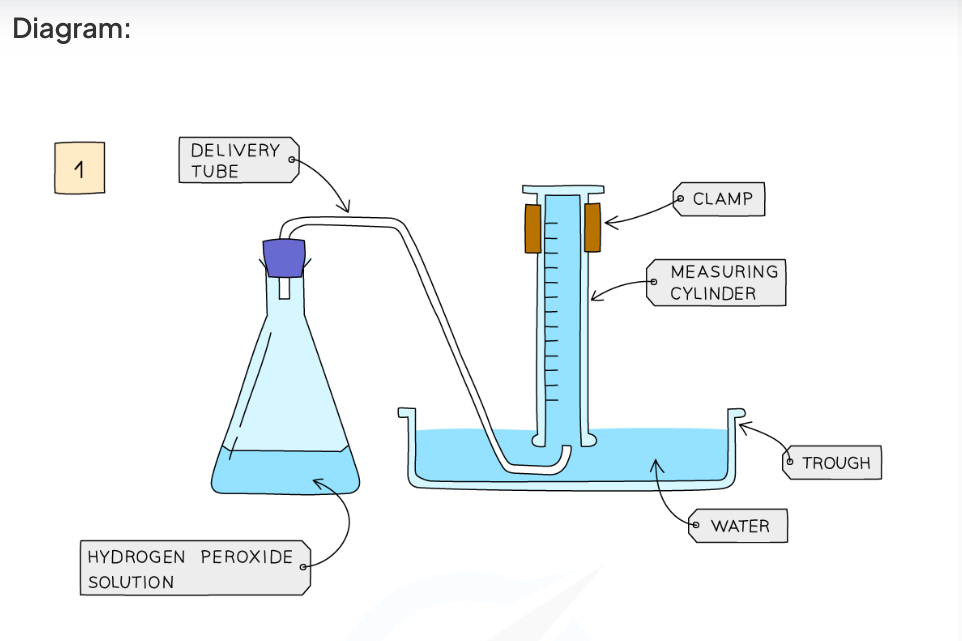
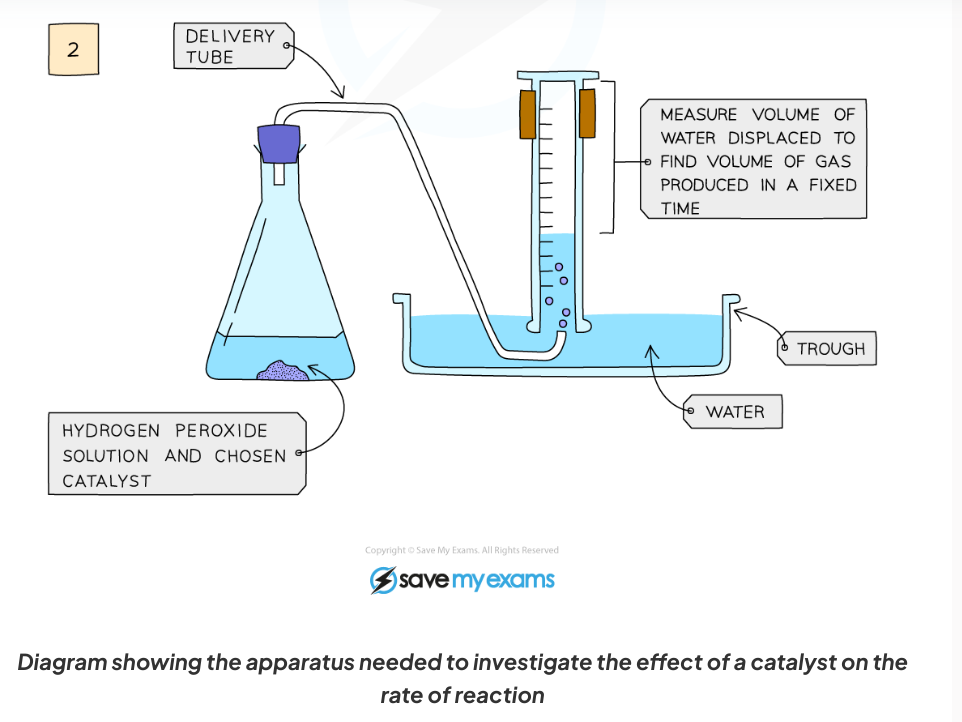
Diagram showing the apparatus needed to investigate the effect of a catalyst on the rate of reaction
Method:
Add hydrogen peroxide into a conical flask
Use a delivery tube to connect this flask to a measuring cylinder upside down in water trough
Add the chosen catalyst into the conical flask and close the bung
Measure the volume of gas produced in a fixed time using the measuring cylinder
Repeat experiment with different catalysts and compare results
Catalysts to try could include: manganese(IV) oxide, lead(II) oxide, iron(III) oxide and copper(II) oxide
Result:
The data for different catalysts can be plotted on the same graph and the relative rates compared