Module 7 - Cytoskeleton
1/96
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
97 Terms
LEC 1
Recall the cell’s cytoskeleton, what are the fibers it’s composed of?
-microtubules (tubulin)
-microfilaments (actin)
-intermediate filaments AKA IFs
myosin, dynenin, and kynesin are motor proteins that move along the fibers (actually only microtubules and microfilaments, not IFs).
What filament will we focus on in this lecture?
Actin!
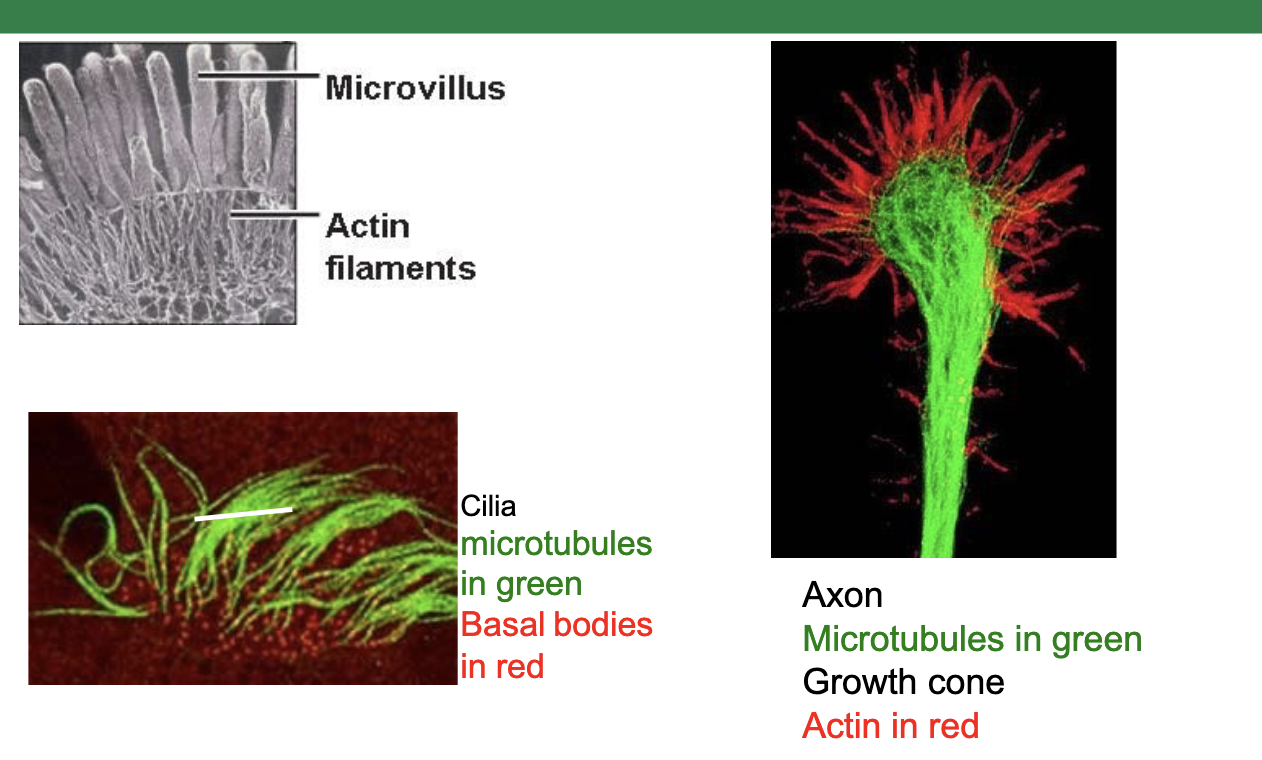
e.g. in this neuron on the right, we see microtubules (shown in green) providing structure to the elongated axon, while actin (shown in red) provides shape and function to the growth cone
actin filaments AKA…
…AKA F-actin!
For a cell that migrates, it will express [actin-GFP / tubulin-GFP] at the time.
For a cell that divides by mitosis, it will express [actin-GFP / tubulin-GFP] at the time.
For a cell that migrates, it will express actin-GFP.
For a cell that divides by mitosis, it will express tubulin-GFP.
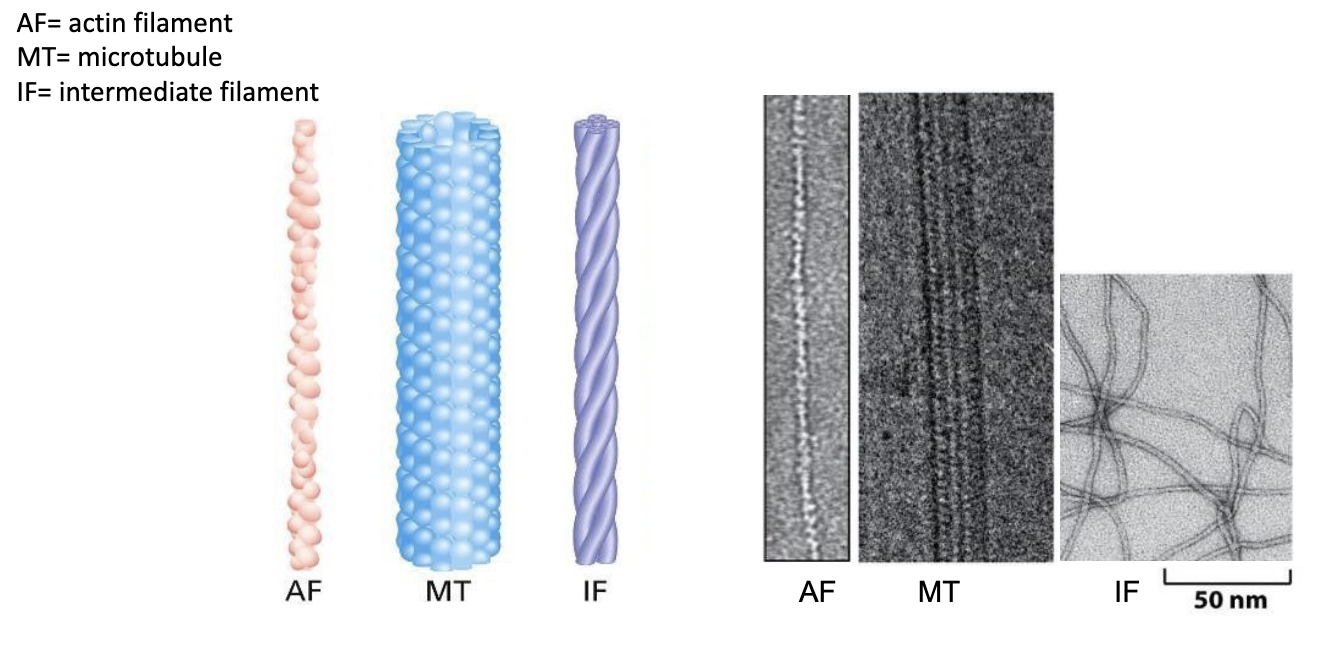
T/F: Actin filaments are the thinnest of the cytoskeletal fibers.
true!
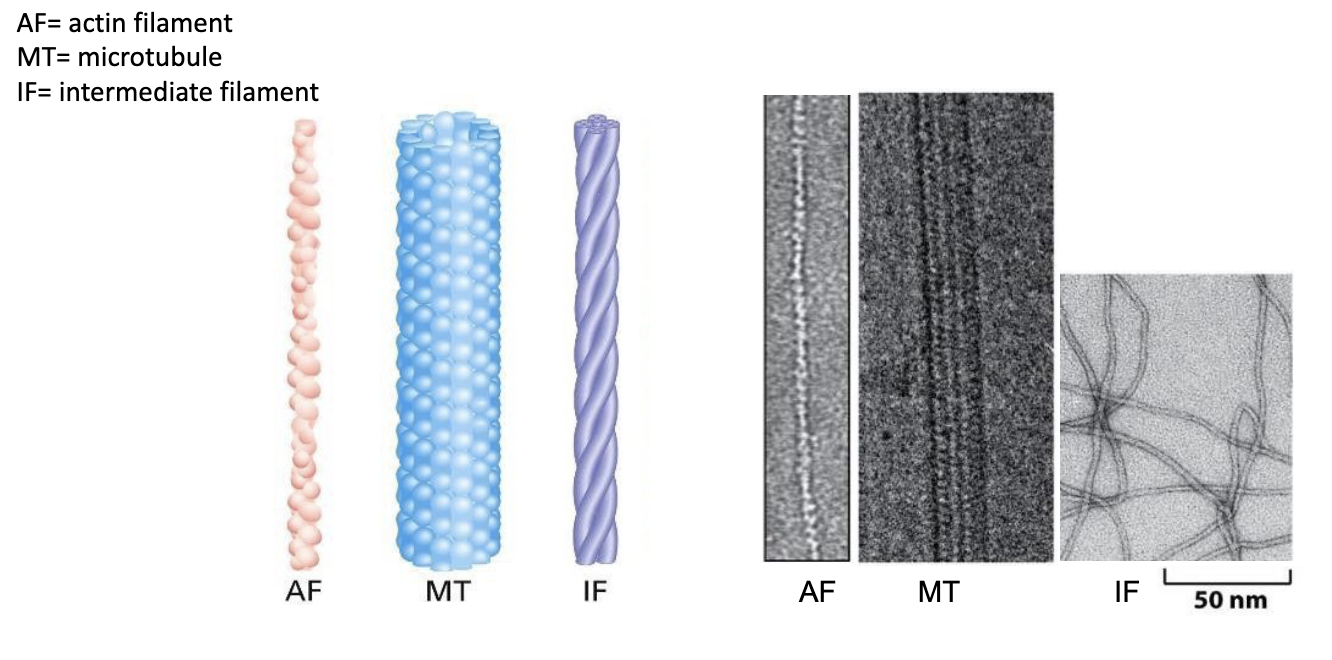
T/F: Microtubules are the thickest fibers.
true!
Myosin is a motor protein that moves along actin (micro)filaments. Dynein and kinesin are motor proteins that move along tubulin (micro)tubules.
What moves along intermediate filaments?
nothing lol! trick question. no motor proteins have been identified to be associated with intermediate filaments
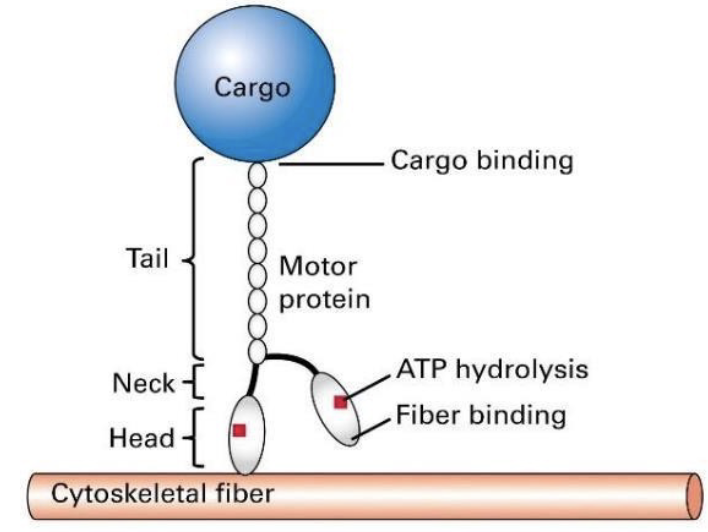
What is the general structure of a motor protein?
-they step along their respective fibers using cycling chemical reactions.
-the head domains bind to a cytoskeletal fibre (that is actin filaments or microtubules) and the tail domain attaches to a cargo
-ATP hydrolysis provides energy for these motor proteins to move, or “walk”
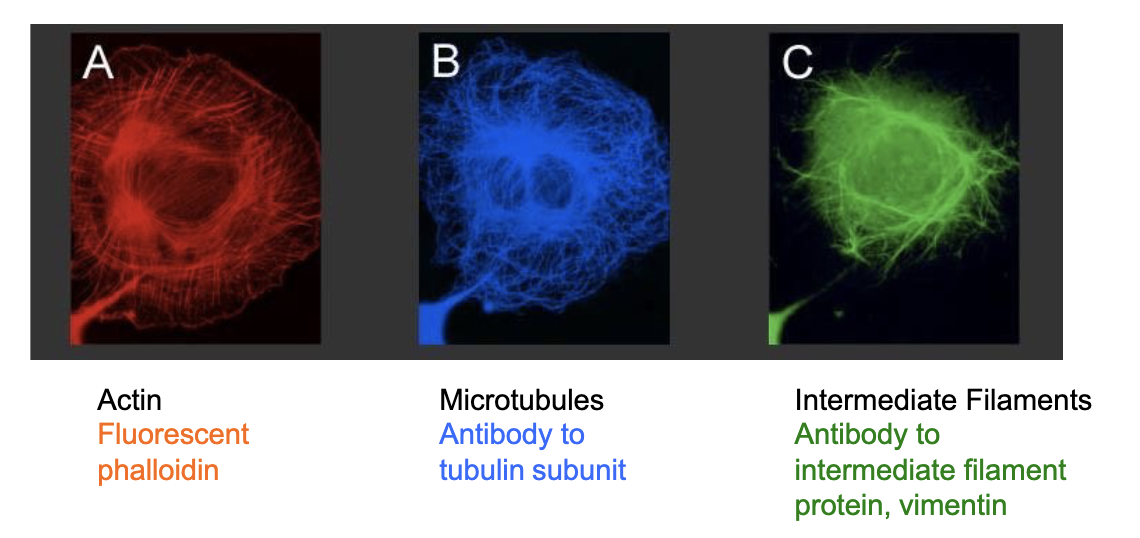
What fluorescent tags are required to visualize actin, microtubules, and intermediate filaments respectively?
actin
reqs. phalloidin molecule
a toxin derived from death cap (a mushroom)
binds to actin with high affinity and specificity; also stabilizes it
microtubules
reqs. antibodies specific to the tubulin or a fusion tubulin-GFP
intermediate filaments
reqs. antibodies specific to the IF or a fusion ___-GFP
Microtubules are made up of [monomeric / dimeric] subunits called ..
Microtubules are made up of dimeric subunits called alpha α and beta β tubulin.
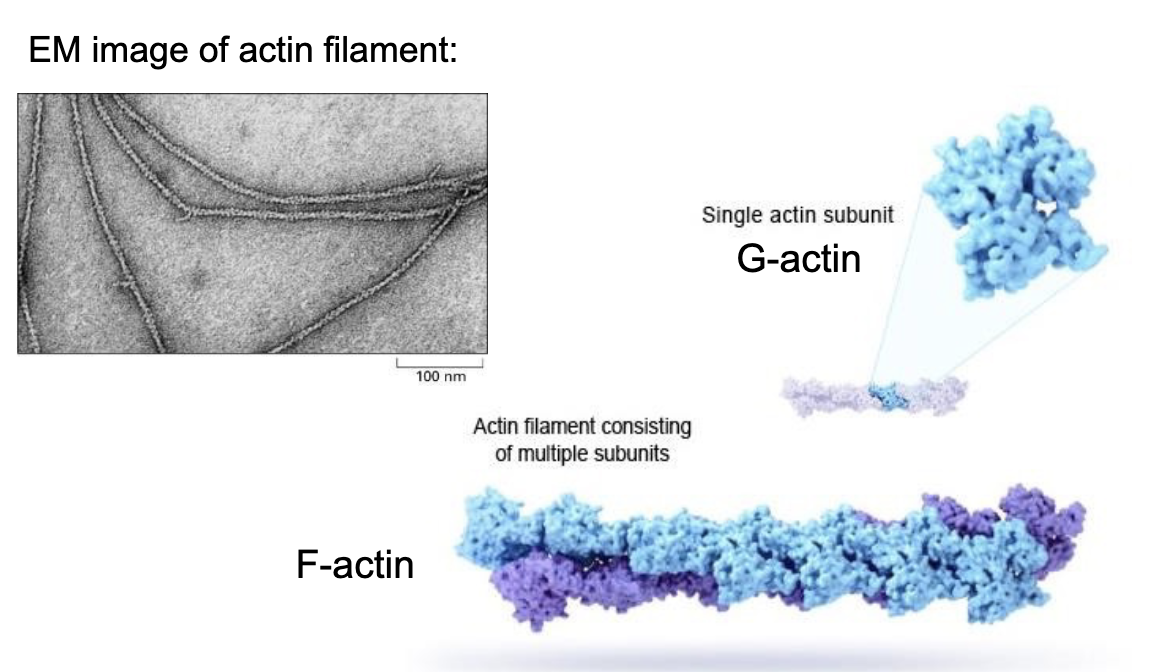
Actin is made up of individual monomeric subunits called..
….G-actin! (globular actin)
which assembles into long helical structures.
Where is the highest density of actin in a cell?
at the cell periphery!
why? cuz of what it does which is help move the cell around (and ofc the feet or podocytes will be at the cell’s periphery).
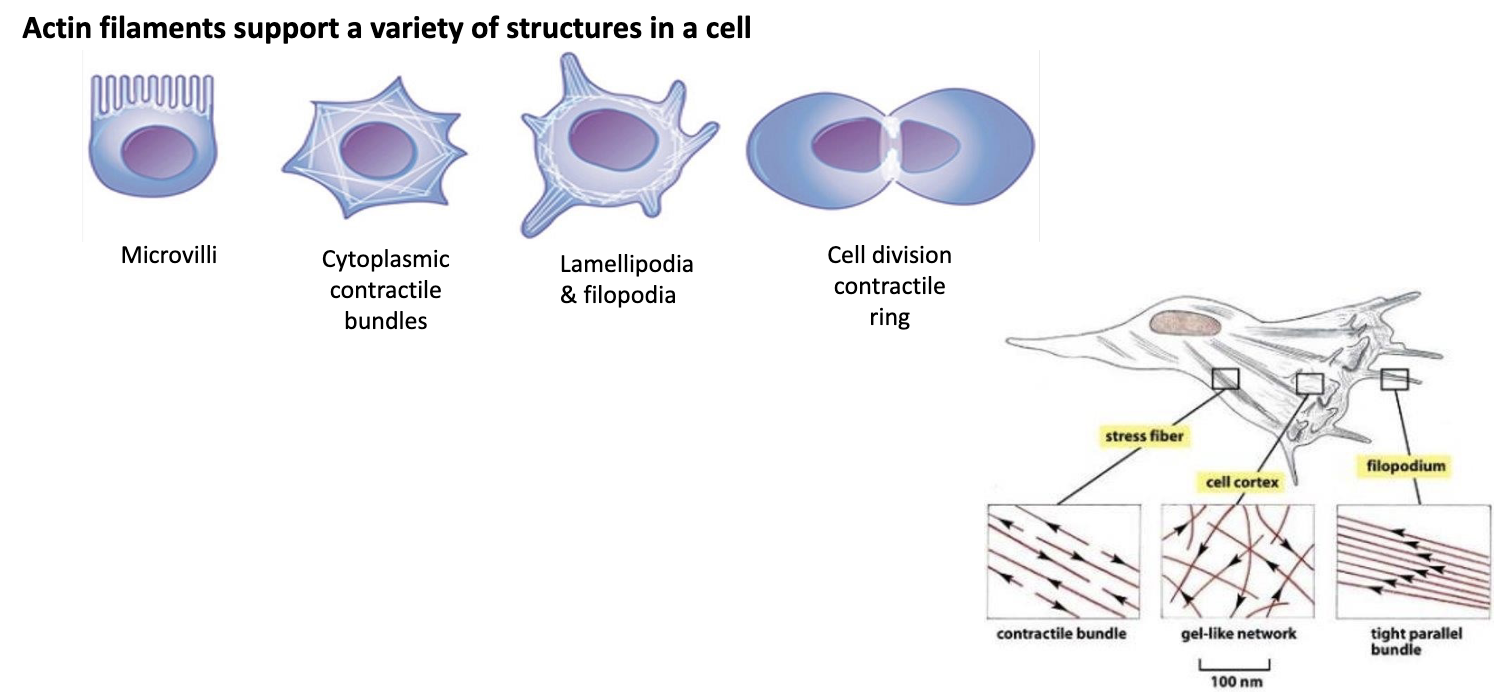
What does actin do?
Hint: it can do a variety of things!
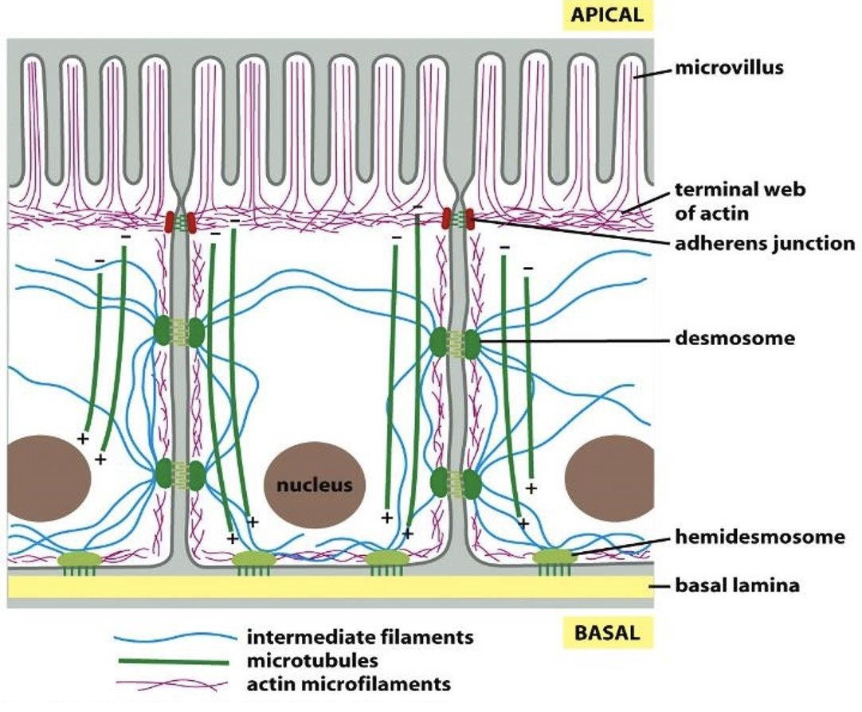
actin filaments (or F-actin) underlies the cell membr. to dictate its shape and cell surface movement.
F-actin is what is responsible for:
-establishment of microvilli
-formation of contractile bundles in muscle cels
-formation of filopodia and lamellipodia needed for cell migration
-formation of contractile ring that directs cell division
Actin filaments have polarity. What does that mean?
a positive end and negative end
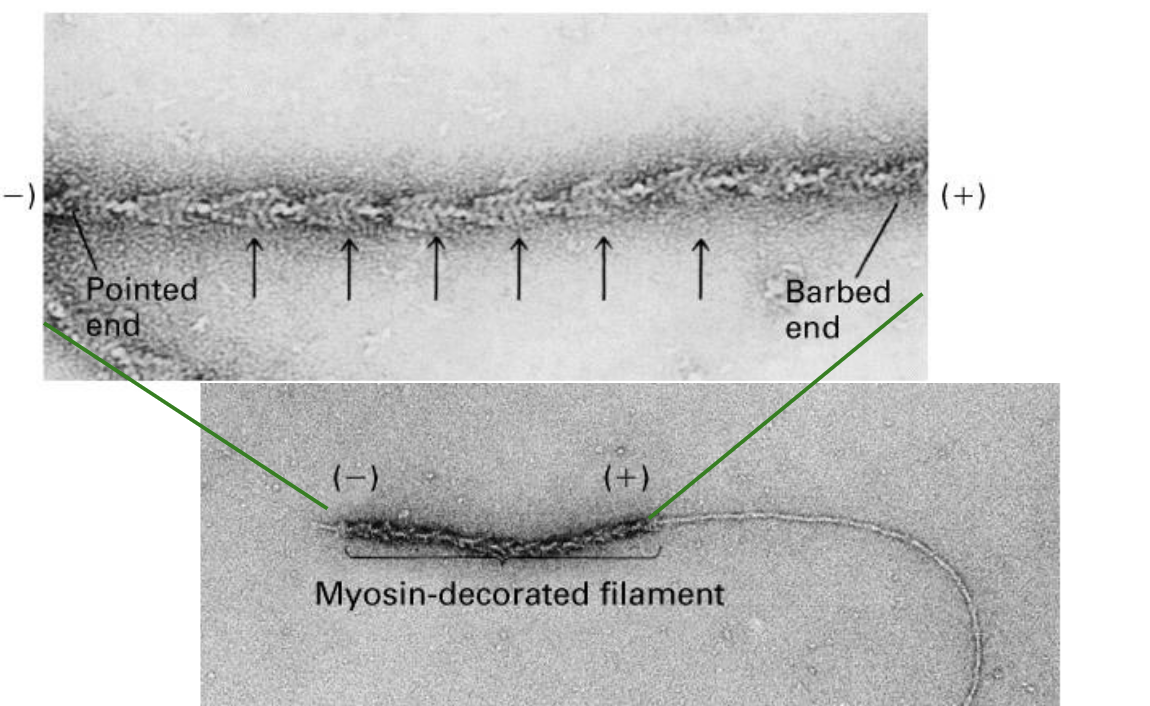
The plus end of an actin filament grows …
… grows more quickly (has more actin subunits added to it) and has a barbed appearance.
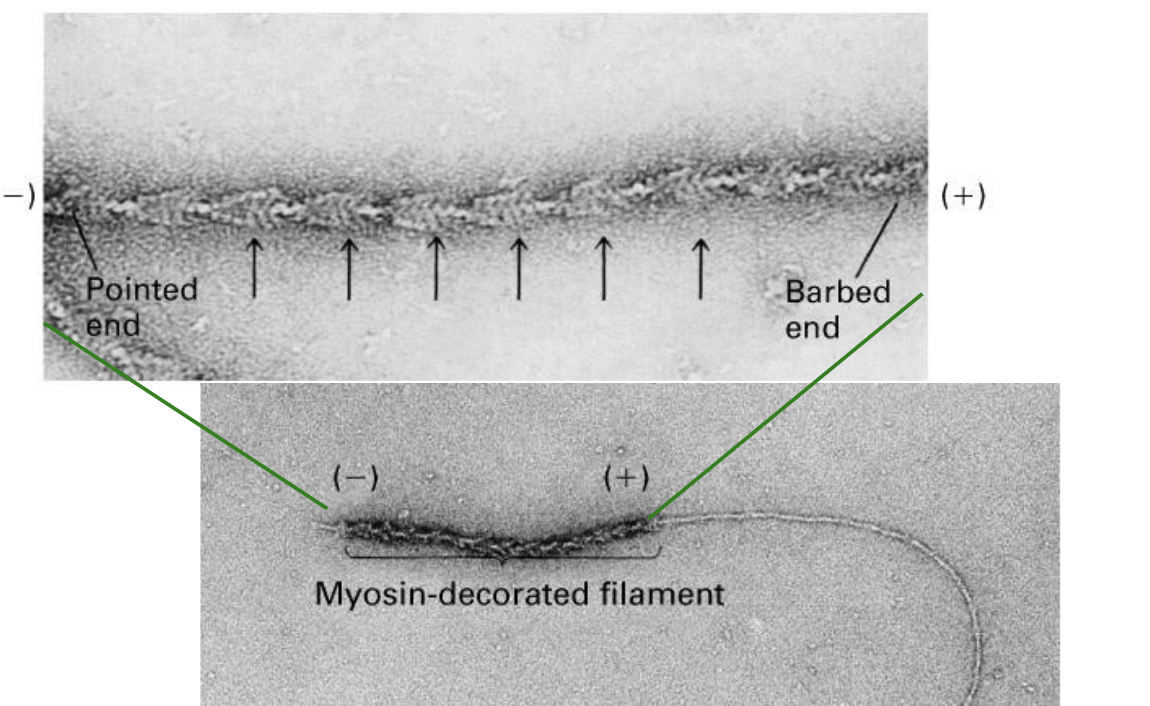
The minus end of an actin filament grows…
… grows slower and may shrink, and has a pointed appearance.
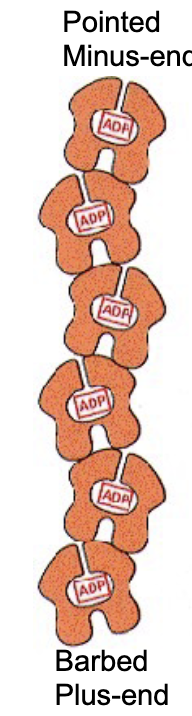
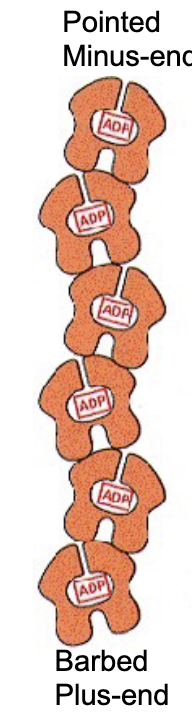
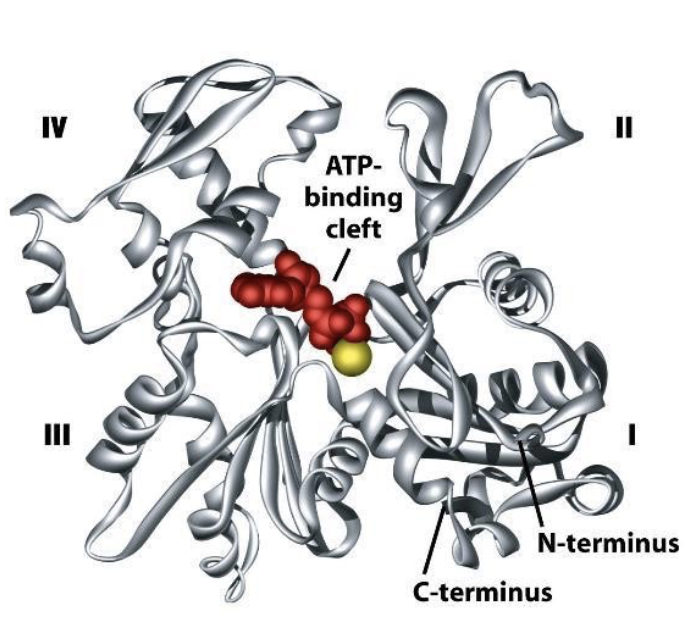
What does a single actin monomer (G-actin) look like?
a globe with 4 structural domains. w/ a cleft btwn domains 2 and 4.
what does the cleft do?
it forms an ATP nucleotide binding site!
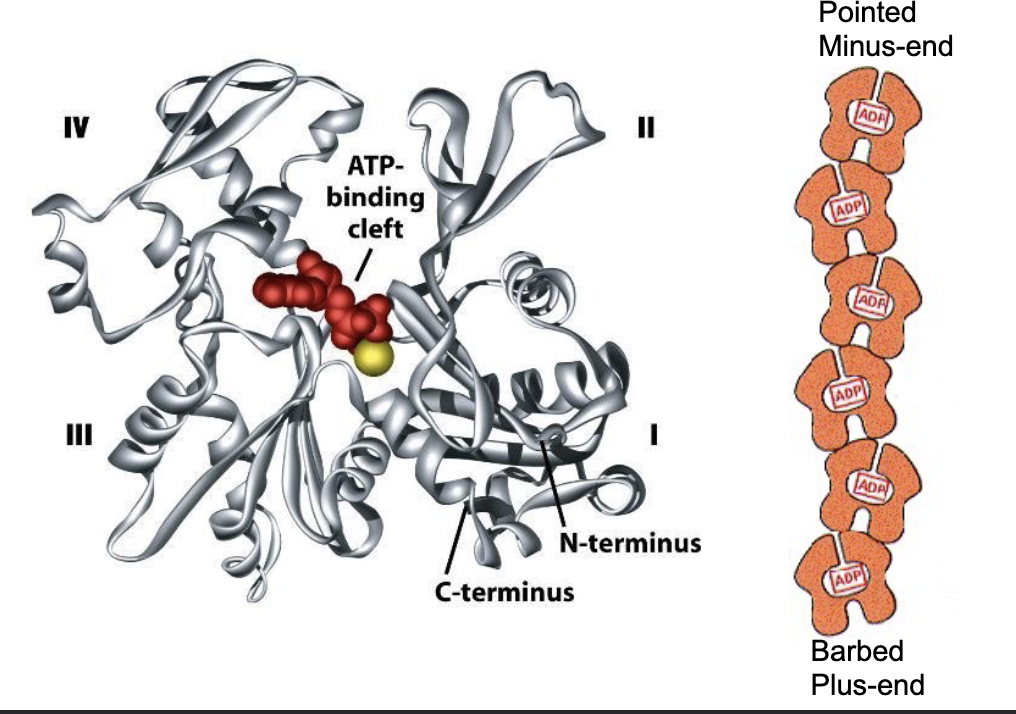
The ATP-binding pocket is pointed to the minus end of the elongating polymer, so that…
…so that the ATP‐binding pocket of each monomer is not exposed within a filament, except for a pair of monomers right at the minus‐end of the actin filament.
The polarity of actin filaments helps them grow and shrink. How?
thru polymerization and depolymerization!
individual G-actin subunits add onto the plus end of the filament making it longer, thanks to ATP powering it.
meanwhile subunits can detach from the minus end, shortening it.
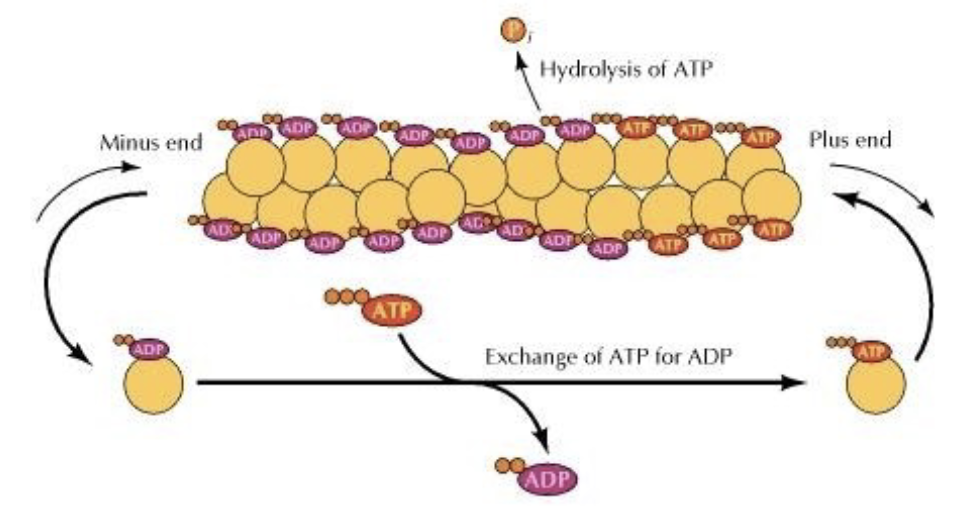
T/F: Technically, polymerization can occur at both the plus‐ and minus‐end
true!
however, there tends to be more growth at the plus‐end and more shrinkage at the minus‐end
G-actin monomers polymerize into…
…into F-actin!
Actin has intrinsic ATP-ase activity. meaning…
it hydrolyzes ATP —> ADP + Pi (inorganic phosphate)
The rate at which growing/shrinking happens depends on the concentration of G-actin available.
What is the tipping point called? What happens at it?
tipping pt is called the CRITICAL CONCENTRATION
-when the rate of polymerization = rate of depolymerization
-if the conc. is above the pt, the filament grows (since rate of poly > rate of depoly)
-below it, it shrinks
T/F: There are also regulatory proteins that can influence the process.
true!
e.g. the protein profilin binds to actin-ATP to promote ATP binding —> increase actin conc. at plus end —> promote polymerization —> make plus end grow
e.g. the protein thymosin binds to actin monomers to inhibit polymerization
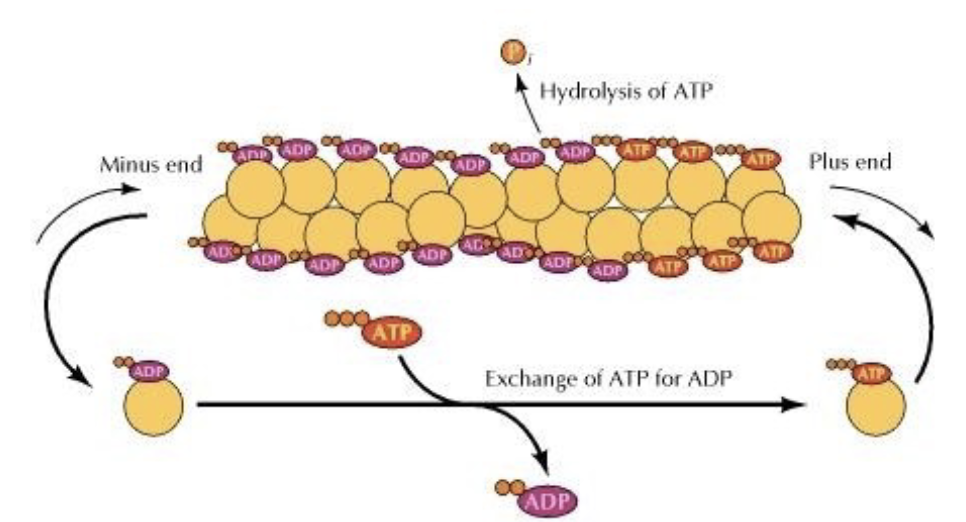
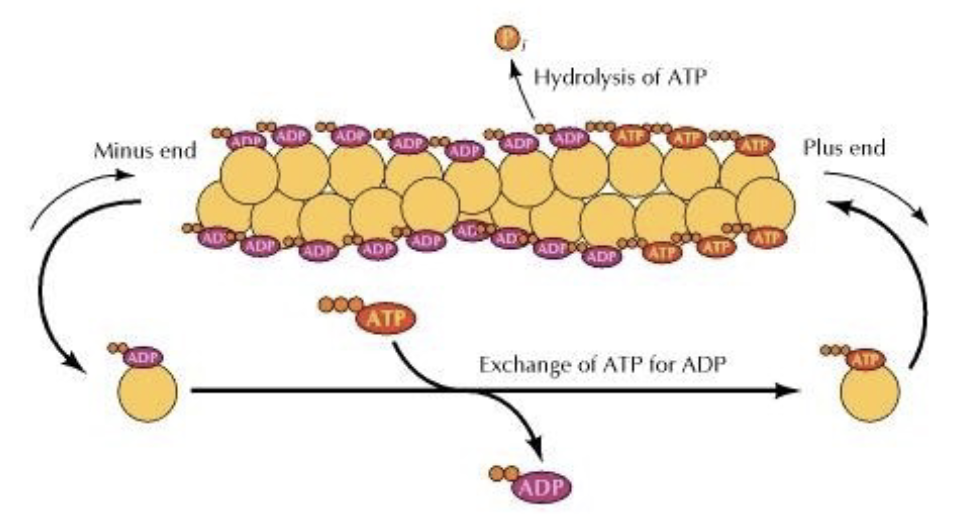
What is TREADMILLING?
treadmilling = forward movement of F-actin but no growth.
why? look at the image. the rate of depoly = rate of poly. so the minus end keeps removing G-actin, which ends up just attaching to the plus end in a constant loop.
How do cells use actin filaments to get around?
They form filipodia and lamealopodia!
they’re actin that push outta the cell membrane.
How do they form?
leading edge is formed (it’s the part of the cell that is closest to the dirn of movement)
fan-like expansions of the membr. = lamellipodia
finger-like “ feet = filopodia
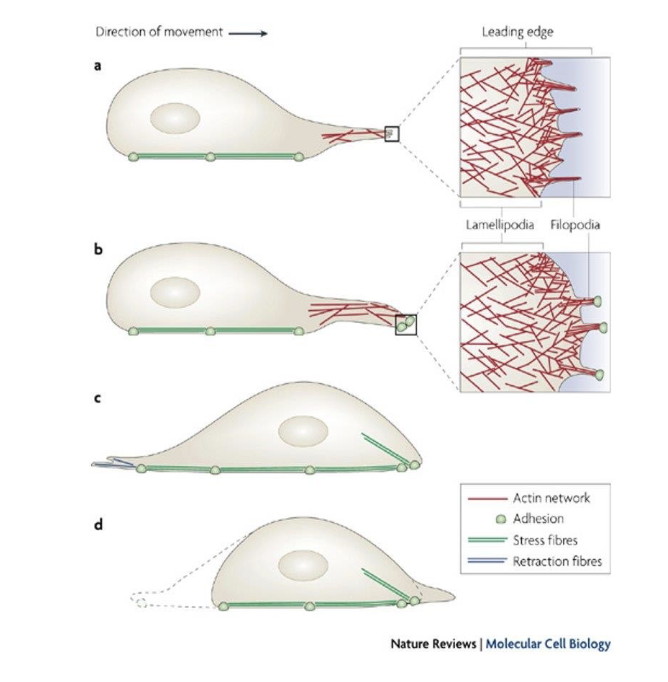
What do filipodia do?
They’re tiny finger like projections driven by the rapid polymerization of actin at the leading edge of the cell.
Analogy: imagine ur cell reaches out w/ tiny fingers to explore its surroundings. that’s what’s filipodia do.
What do lamelopodia do?
they are broader sheetlike extensions that also help the cell to crawl along surfaces. so it’s like the cell is constantly building and extending these little feet to pull itself forward.
Another key player is myosin motor proteins, which use ATP hydrolysis to walk along actin filaments.
How does it work?
they have a head domain (at the N terminus) that binds to actin and a tail domain that can carry cargo.
Most myosin walk towards the [minus / plus] end of F-actin.
Most myosin walk towards the plus end of F-actin.
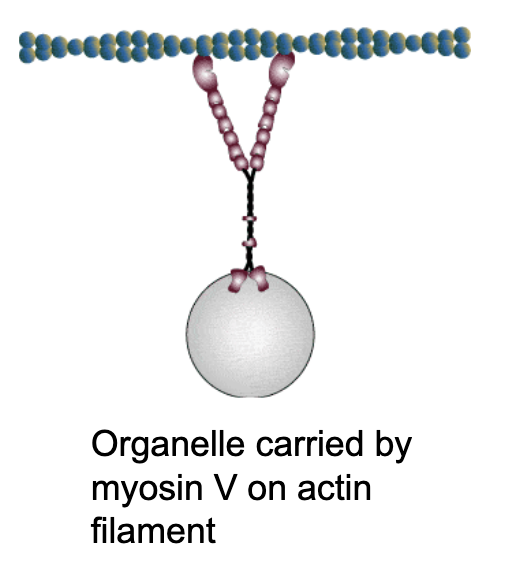
What kind of cargo are these myosin motor proteins carrying?
vesicles filled with proteins, organelles like mitochondria.
What are the 4 family members identified of myosin that we will discuss? there are 8 total
myosin 1, 2, 3 and 5
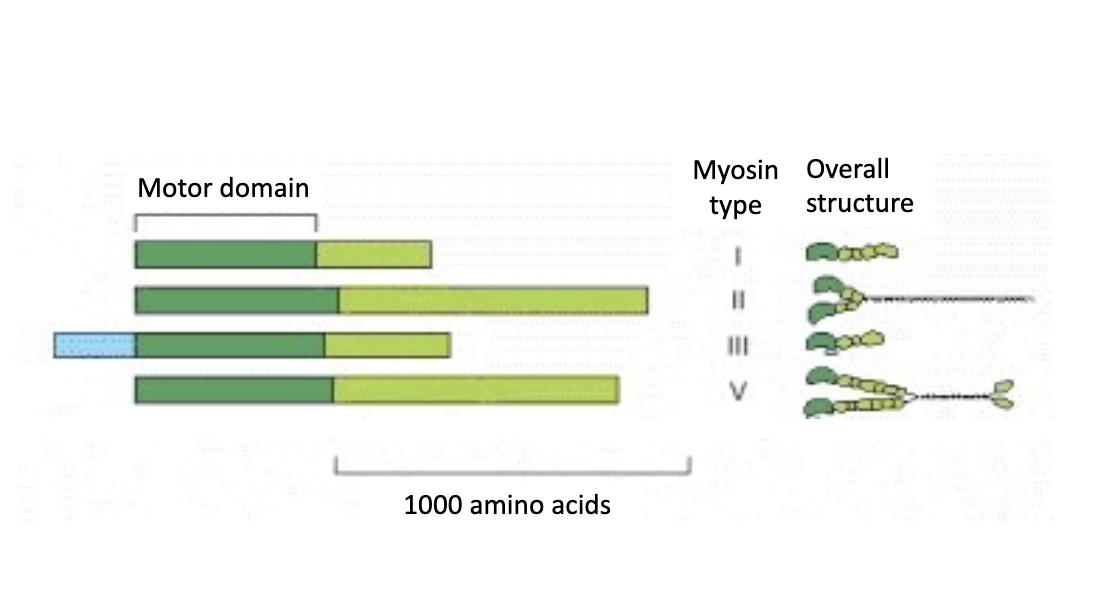
Myosin motors make sure everything gets where it needs to go!
There are different kinds of myosin proteins. What are they?
myosin 2 are responsible for generating contractile forces
myosin 5 transports melanosomes (melanin give our skin and hair color) along actin filaments to ensure pigments are distributed properly
Myosin ___ are the powerhouse behind muscle contraction. They are the most ABUNDANT type of myosin in our bodies.
Myosin 2!
T/F: Myosin 2 carries cargo.
FALSE! myosin 2 doesn’t carry cargo unlike the others.
Rem. anatomy & physio?! In muscle cells, myosin are the thick filaments and actin are the thin filaments, which pull on each other to contract muscle fibers.
just copy anat flashcard + calcium stuff
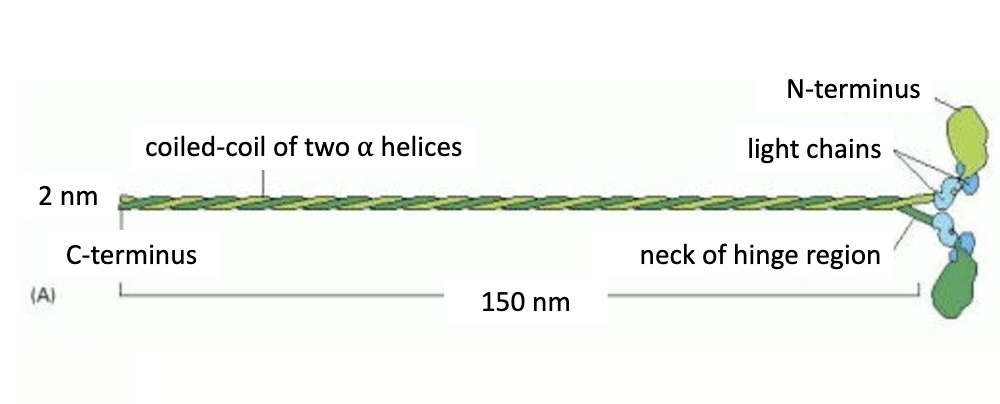
Zoom in on the structure of myosin 2:
Myosin 2:
-2 heavy chains (in green) that form coiled-coil motif
-4 light chains (in blue)
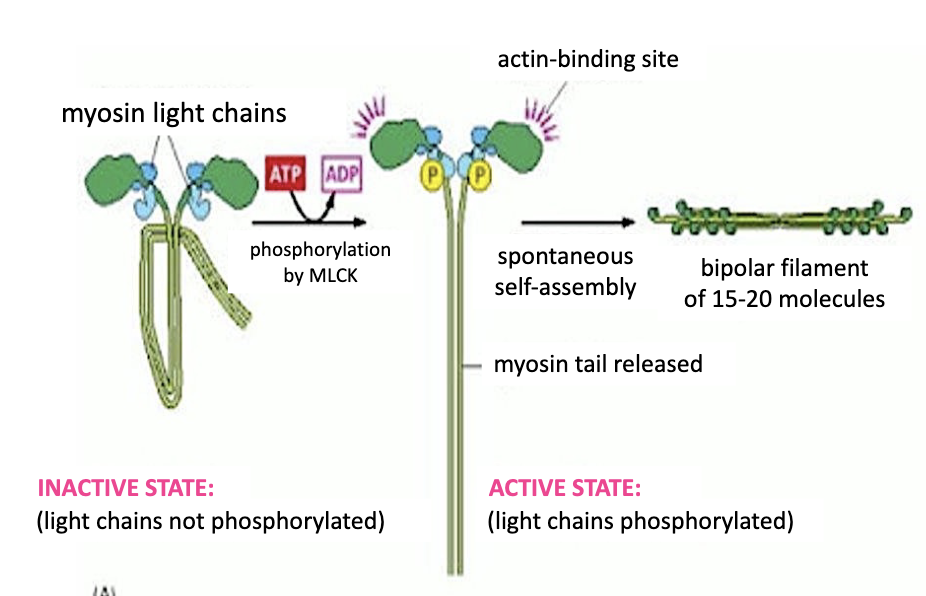
Phosphorylation of myosin light chains of myosin II by ….
Phosphorylation of myosin light chains of myosin II by a myosin light chain kinase (or MLCK), will drive polymerization of the myosin proteins by extending the myosin tails and activating the actin‐ binding domains on the motor heads.
15-20 myosin 2 proteins form a bipolar filament called a MYOSIN 2 THICK FILAMENT.
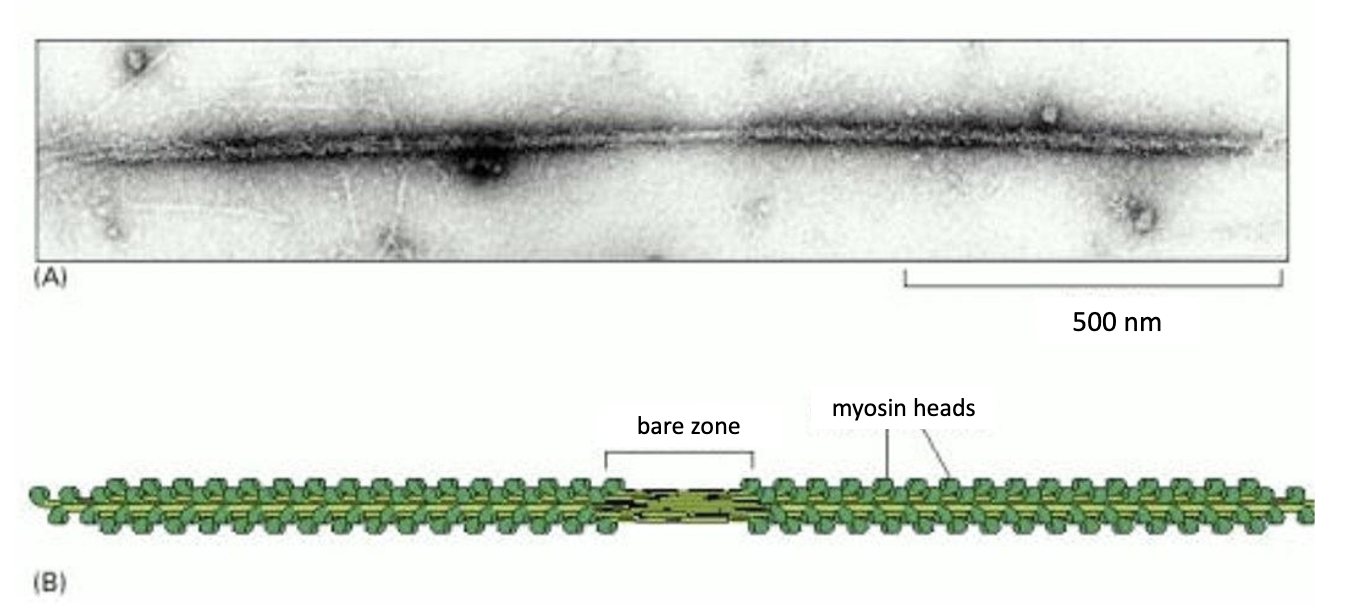
While myosin 2 helps with contractile activities, myosin 5 powers intracellular trafficking of cargo along F-actin.
What is a specific example of this?
Myosin 5 transports melanosomes (whose pigment granules called melanin give our skin and hair color) along actin filaments to ensure pigments are distributed properly
Mutations in myosin 5 can disrupt transport leading to _______________ phenotype in animals.
dilute phenotype!
when pigments aren’t distributed evenly = faded color.
While microtubules are also involved in the transport of melanosomes, myosin 5 distributes the melanosomes…
… to the cell membrane along actin filaments.
What cells do these melanocytes enter into?
Each melanocyte in the epidermis of the skin has several dendrites that stretch out to connect it with many keratinocytes.
Melanin enters into the keratinocytes of our skin cells, and distribution of this pigment at the apical periphery of our skin cells protects the cell’s DNA from UV‐ damage when tanning.
How do myosin 5 move?
via a hand-over-head motion where the trailing head detaches and becomes the leading head in a stepping manner. goes from minus end to plus end. JUST LIKE WHEN WE USE OUR FEET TO WALK.
the lever arm length is the distance by which the power stroke propels the myosin fwd.
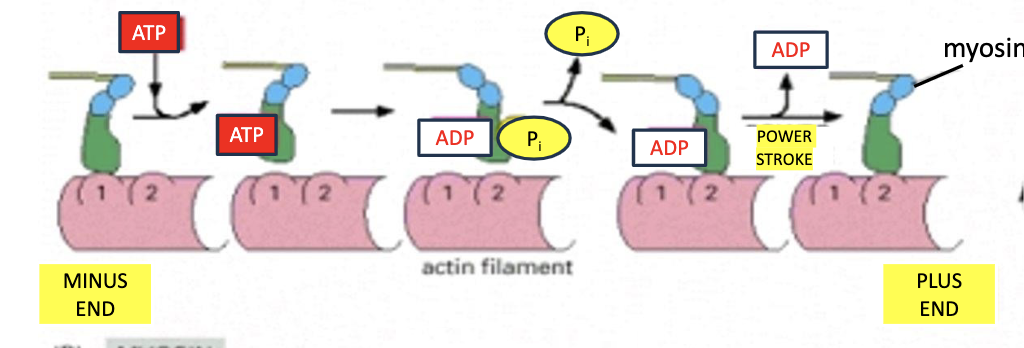
T/F: The rate of myosin motor protein movement is the same for all myosin.
false! they can range from 0.2 - 60 micrometers/sec depending on the myosin protein.
The rate depends upon the cycle of ATP nucleotide binding and hydrolysis and varies with
1) the rate of ATP hydrolysis by ATPase in each myosin head,
2) and the proportion of time myosin is bound to the actin filament, a result of affinity.
![<p>Myosin 5 spends up to 90% of the cycle bound to actin, compared to 5% for myosin 2. <br>Thus myosin 5 will move [faster/slower] along F-actin than myosin 2.</p>](https://knowt-user-attachments.s3.amazonaws.com/be3fa434-5d79-4ce0-a75d-c746ebe978cc.png)
Myosin 5 spends up to 90% of the cycle bound to actin, compared to 5% for myosin 2.
Thus myosin 5 will move [faster/slower] along F-actin than myosin 2.
Thus myosin 5 will move slower along F-actin than myosin 2.
What is the step size of myosin 5 vs myosin 2?
The myosin V lever is three times longer than that of myosin II. As a result, the step size of myosin II is about 7 nm compared to myosin V which takes about 36‐nm steps.
LEC 2
Now we will discuss the other element of the cellular cytoskeleton: microtubules.
…
What are microtubules made of?
microtubules are hollow tubes. where protofilaments make up the tube wall (via a circular pattern), and protofilaments are made up of alpha and beta tubulin proteins linked up (heterodimers).
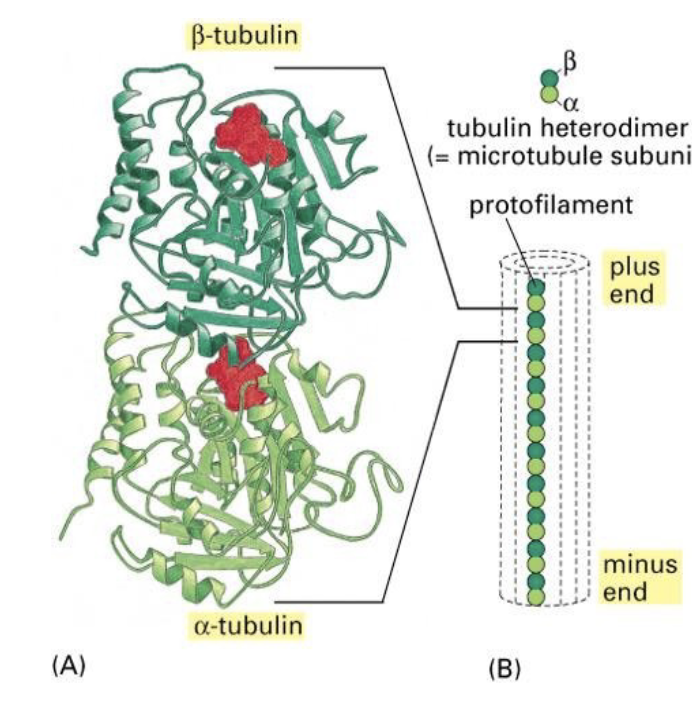
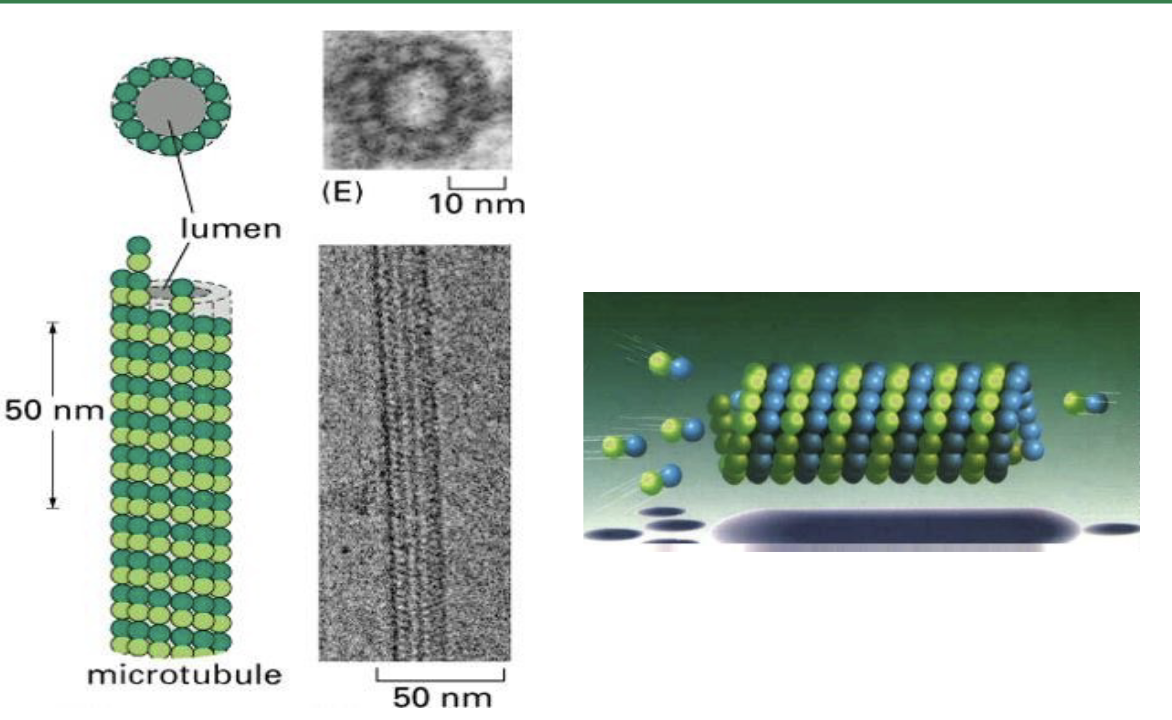
The protofilaments are staggered so that if you follow a string of tubulin monomers, …
The protofilaments are staggered so that if you follow a string of tubulin monomers, they appear to spiral up through the microtubule like a spring.
What binds the tubulin protein together in the protofilament chain?
GTP! GTP binds to the tubulin proteins, and when it’s bound, they love to stick tg.
analogy: GTP is like the glue here.
But if GTP is always bound, wouldn’t the microtubules just grow forever?
well GTP doesn’t stay bound forever so don’t worry!
GTP will obviously break down into GDP + Pi via hydrolysis.
Is the alpha or beta tubulin subunit more tightly bound to GTP?
alpha!
The GTP that is bound to α‐ subunit is never hydrolyzed and does not exchange with nucleotides in solution. Whereas GTP bound to the β‐subunit is cyclically hydrolyzed to GDP. From there, the GDP is exchanged for GTP.
T/F: Just like actin filaments, microtubules are also polar filaments.
true!
-and again, the plus‐end is the fast‐growing end, while the minus‐end is the slow‐growing end.
-the tubulin dimers are oriented so that the β‐ subunit is closer to the plus‐end, and the α‐subunit is closer to the minus-end.
-Dimers containing α‐β‐GTP are added to the plus end of a growing filament (also referred to as the rescue phase). In contrast, dimers containing α‐β‐GDP are released from a shrinking filament (also referred to as catastrophe).
What is a catastrophe? What is a rescue?
when α‐β‐GTP is added to plus end = rescue
when α‐β‐GDP is removed from minus end = catastrophe
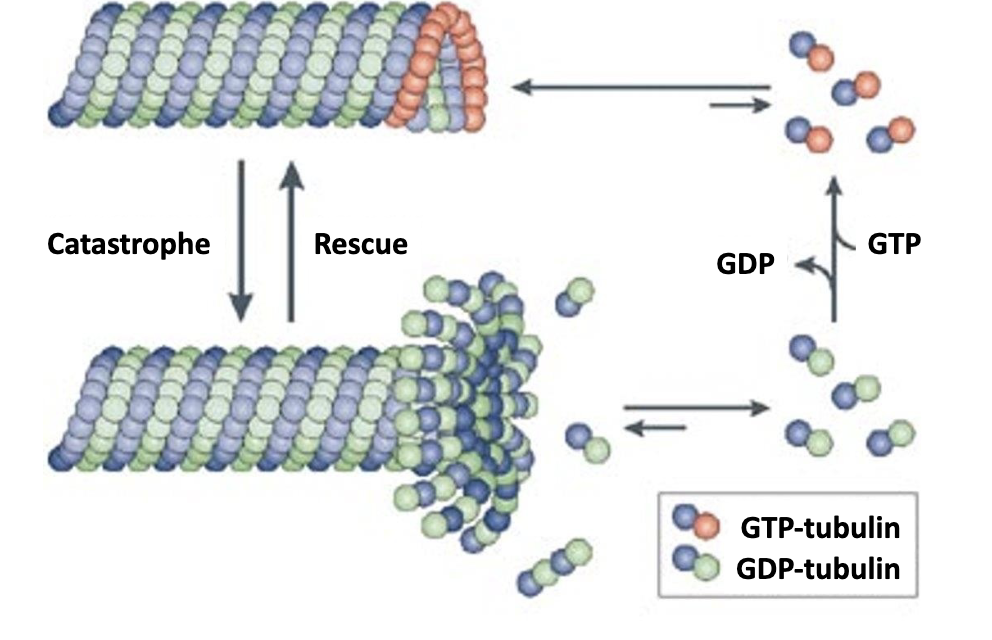
Most of the microtubule consists of dimers containing [α‐β‐GDP / -GTP]?
Most of the microtubule consists of dimers containing α‐β‐GDP. since GTP hydrolysis occurs within the polymerized microtubule
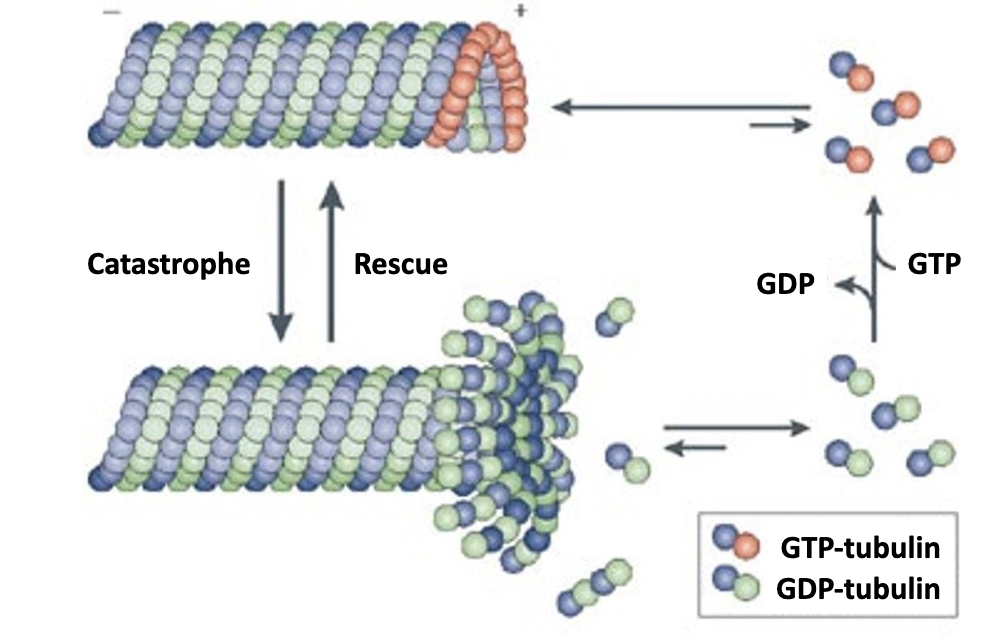
A α‐β‐GTP cap at the plus end favors [growth / shrinkage ].
A α‐β‐GTP cap at the plus end favors growth!
Why? cuz α‐β‐GTP has a higher affinity than -GDP for microtubules. so α‐β‐GTP dimers have four times slower dissociation rate compared to an α‐β‐GDP dimer.
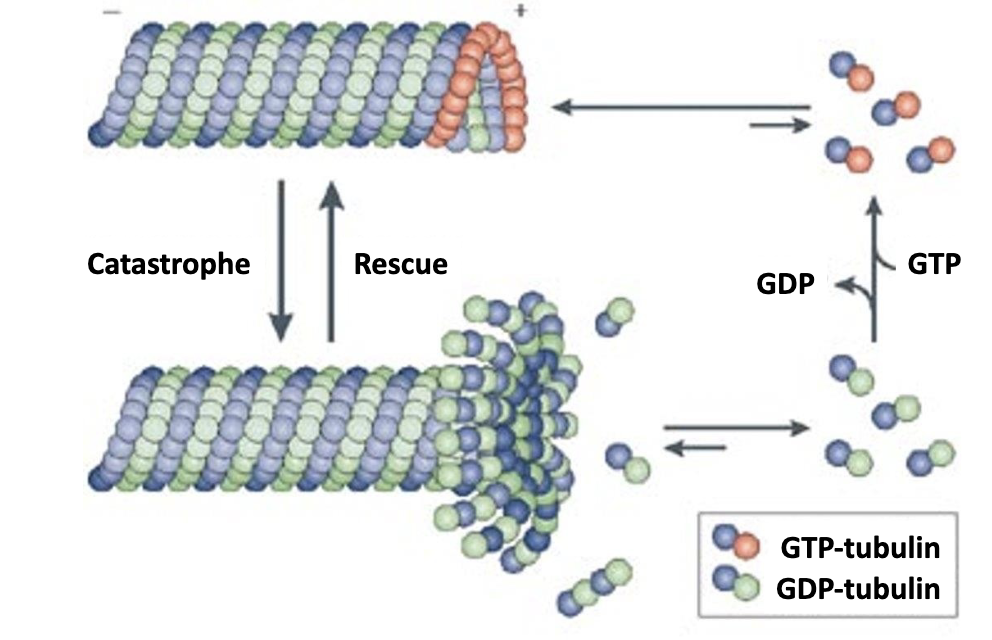
So the growing/shrinking happens again for these proteins.
Lots of GDP —> strong bonds —> microtubule grows
GTP gets broken down —> bonds weaken —> …
—> microtubule shrinks!
Microtubules also have distinct ends: a plus and a minus end.
What do these ends allow for?
they allow for directed movement within the cell!
What is an example of a positive regulator of microtubule growth? and thus stability
EB1!
it is a plus-end binding protein that prevents premature catastrophes and positively regulates growth.
ex. EB1, Tau.
What is an example of a negative regulator of growth? and thus instability
e.g. Catastrophin
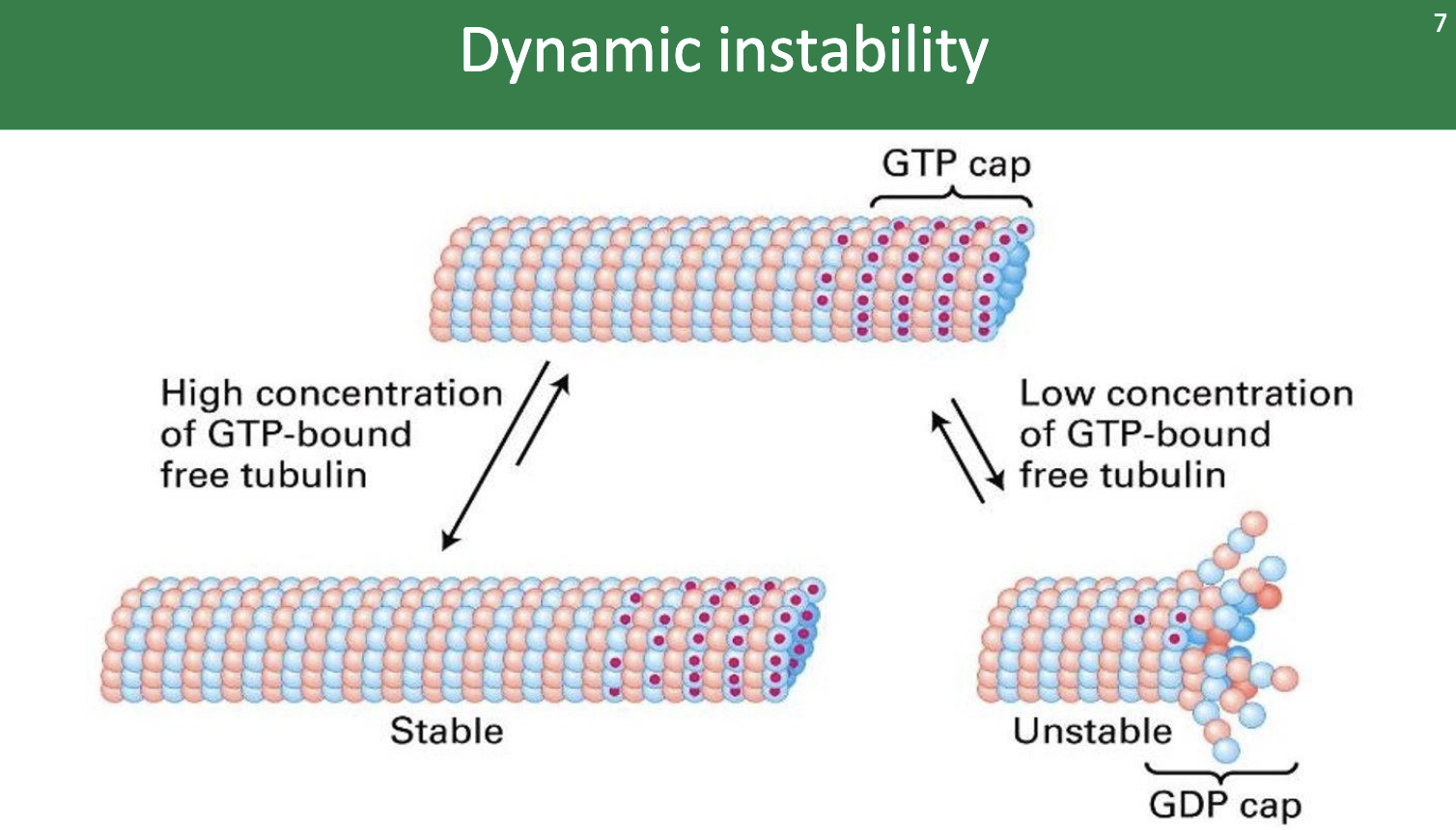
The plus end of the microtubule shows dynamic instability. What does that mean?
dynamic instability = an oscillating behavior between growth (AKA polymerization or rescue) and shortening (depolymerization or catastrophe)
The concentration of free α‐β‐GTP dimers is maintained at a level that …
… that allows polymerization!
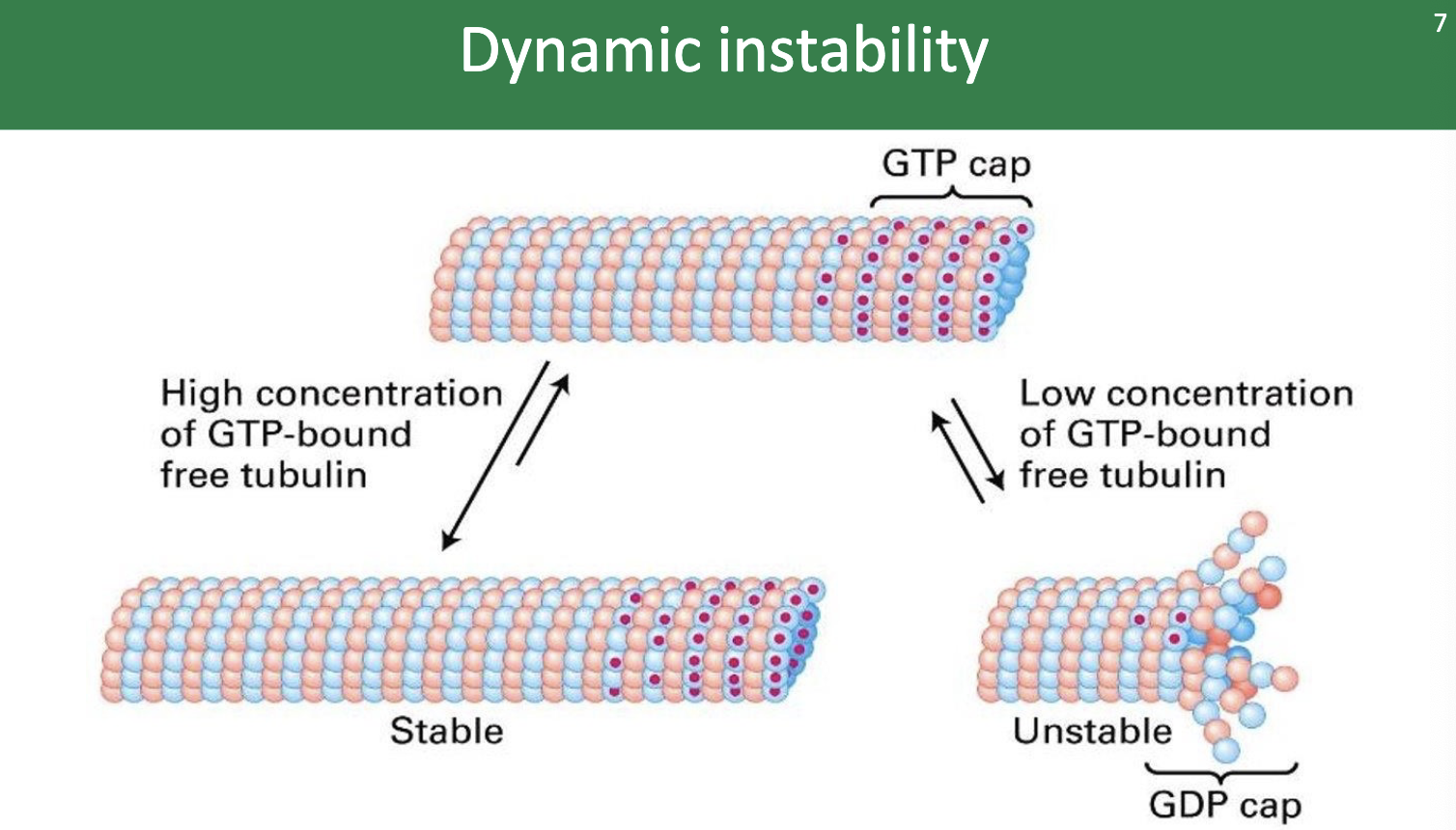
Growth AKA polymerization AKA
.. rescue phase!
Shortening AKA depolymerization AKA
… catastrophe phase!
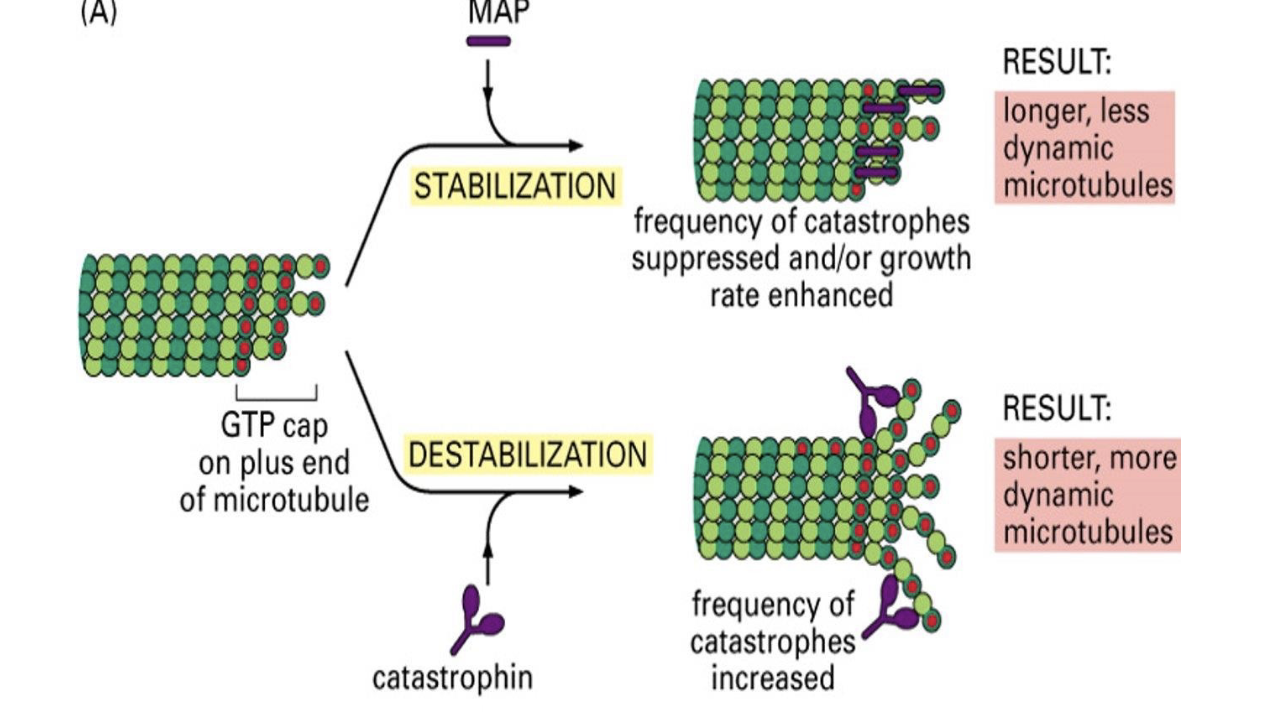
Microtubule Associated Proteins =
=MAPs
What do MAPs do?
MAPs control assembly and disassembly of microtubules!
They’re classified into those that STABILIZE versus those that DE-STABILIZE the filament.
While α‐ and β‐tubulin are the building blocks of microtubules, another type of tubulin, __‐tubulin, is involved in nucleation (or starting off the growth) of microtubules.
γ‐tubulin or gamma tubulin!
T/F: Gamma tubulin is present in much smaller amounts in cells than alpha or beta tubulin.
true!
What does gamma tubulin do?
γ‐ tubulin and other associated proteins form the γ‐ tubulin ring complex or γ‐TuRC.
This ring nucleates at the minus‐end of a new microtubule by forming a template for the growing, plus‐end. Overall, γ‐ TuRC acts as a cap of the minus end while microtubule growth and dynamics occur at the plus end
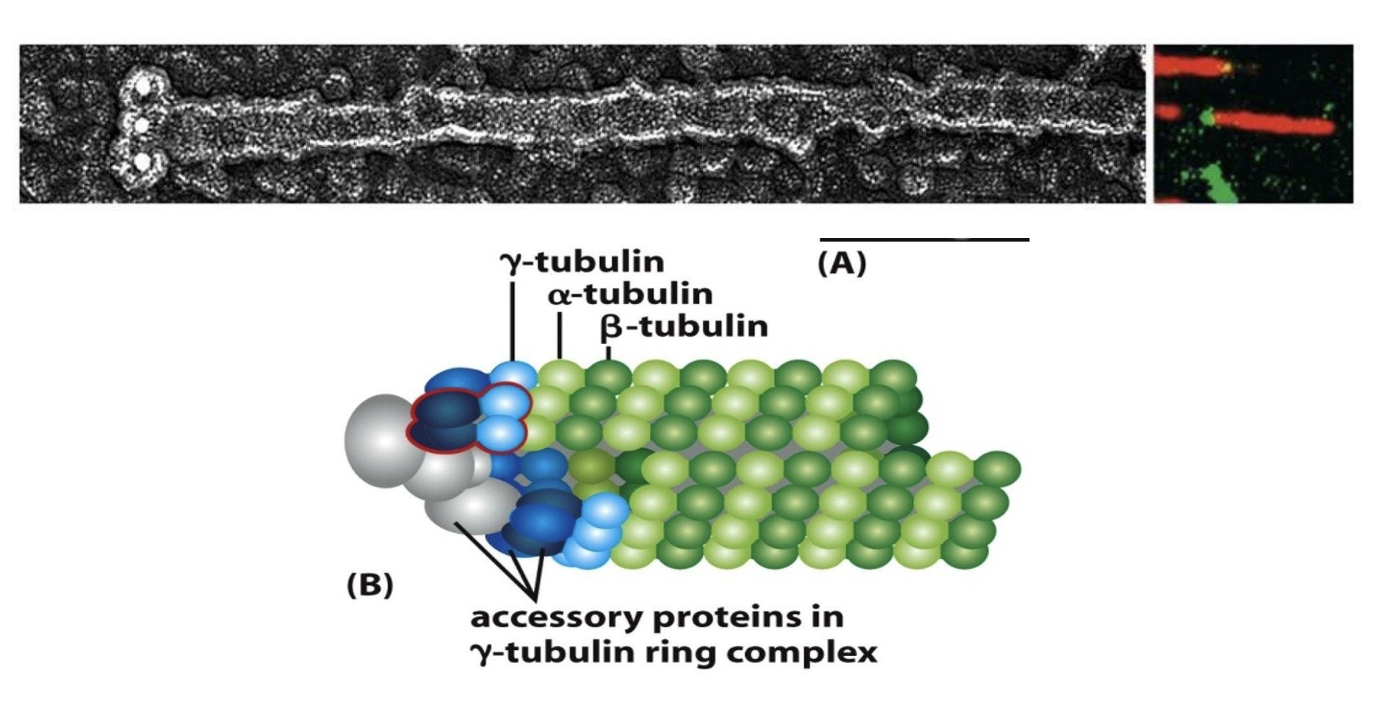
Where do microtubules even come from (i.e. where do they start growing in the cell)?
the microtubule organizing center (MTOC)!
The MTOC (black arrow) has 2 cylindrical structures called centrioles + a cloud of pericentriolar matl (PCM) that contains multiple γ‐ TuRC complexes (in red circles).
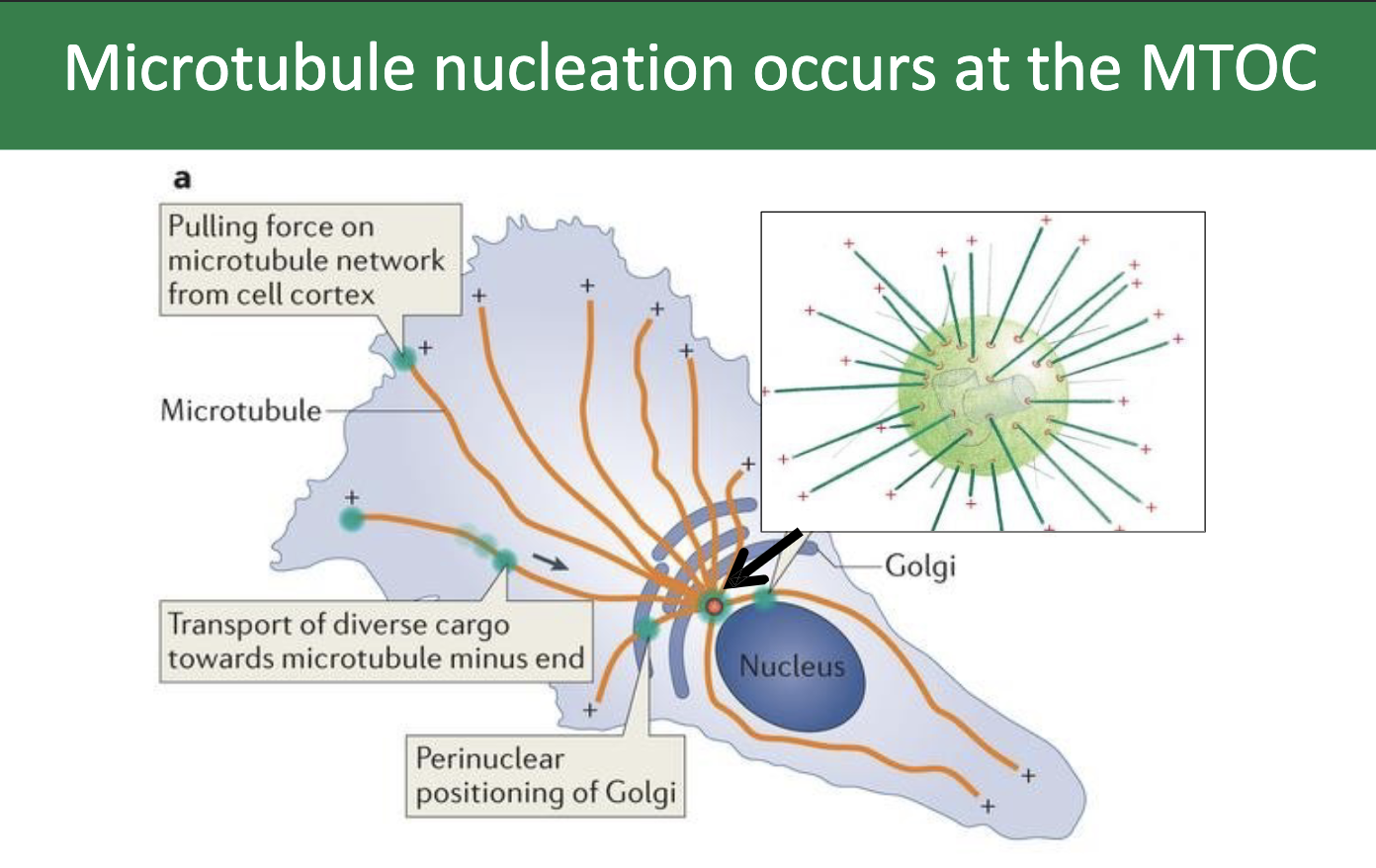
What is the directionality of the microtubule?
The minus‐ends of the microtubules are nucleated at the γ‐TuRC complexes.
Microtubule plus ends are directed towards the periphery of the cell, and are noted here as the plus symbols
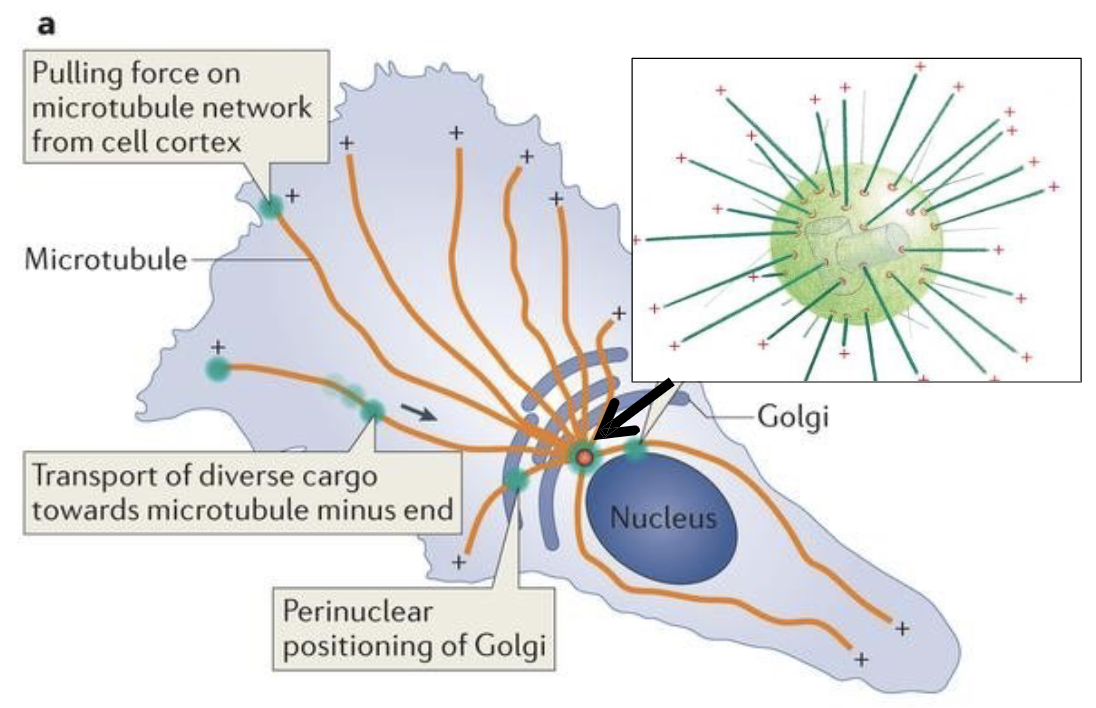
In animal cells, the MTOC is called the …
…the centrosome!
What’s inside the centrosome?
the 2 centrioles!
View of the MTOCs and microtubules during mitosis:
…
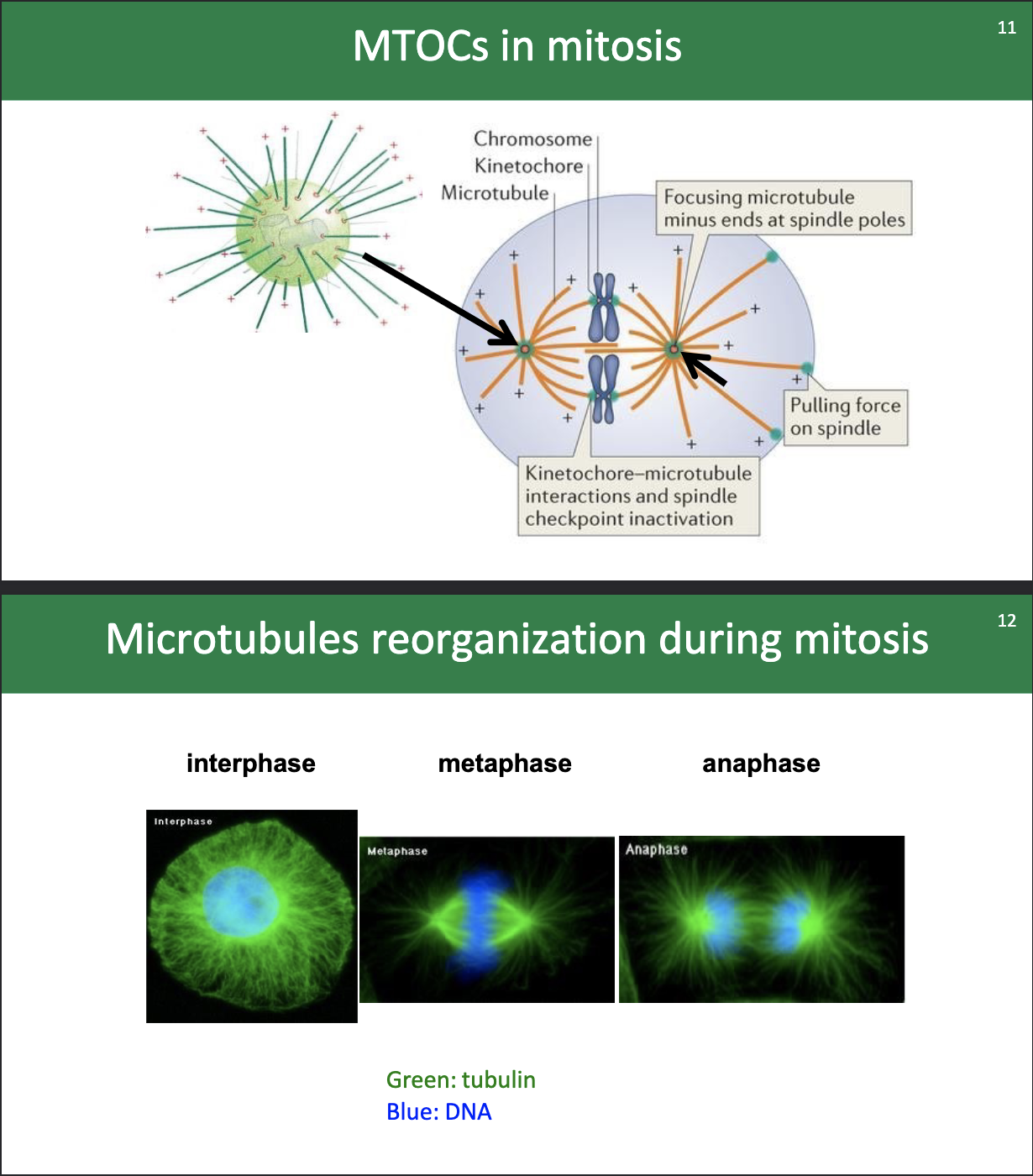
Why would scientists want to use drugs to mess with microtubulin dynamics?
certain drugs can bind to tubulin and can either prevent microtubules from forming or prevent them from breaking down!
this can help us understand how microtubules function in all kinds of cellular processes!
A drug called Colchicine comes from what plant?
What does it treat?
Colchicine comes from the Autumn crocus plant.
it’s been used for ages to treat gout.
why?
cuz it binds to alpha beta tubulin —> prevents microtubules from polymerizing further
Cells in mitosis that are treated with colchicine will …
… will arrest in metaphase without chromatid separation.
A different drug called Taxol comes from what plant?
What does it treat?
Taxol comes from pacific yew tree!
it can treat cancer.
why?
it has the opposite effect of Colchicine, where it actually prevents tubules from breaking down (and thus prevents disassembly of microtubules and thus cell division). so it can disrupt cancer cells that rapidly divide.
Let’s talk about the motor proteins involved in microtubules.
What are the two?
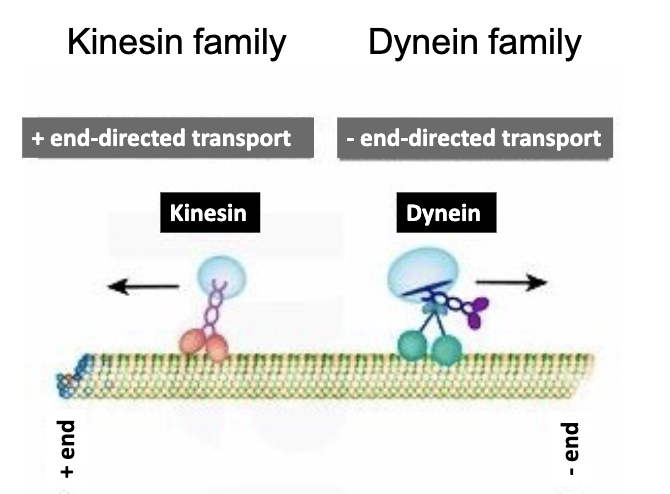
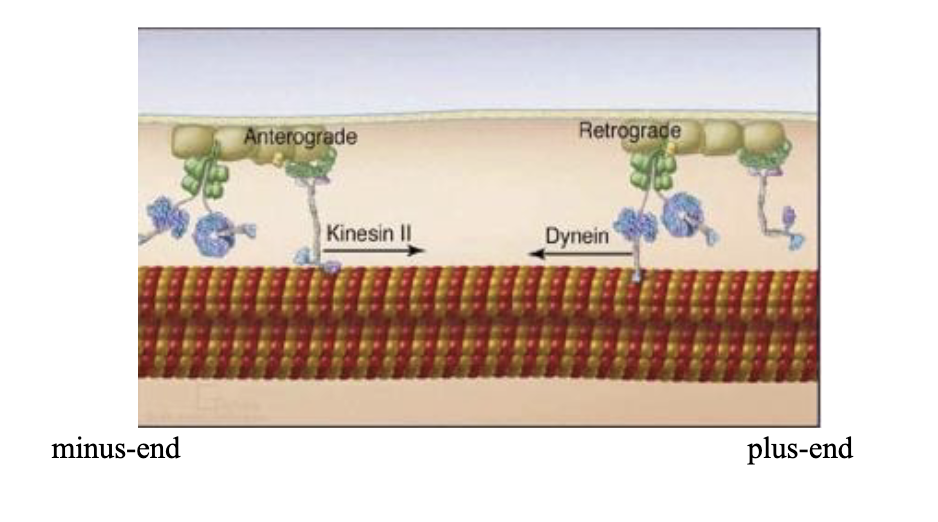
Kinesin and dynein!
Kinesin walks towards the [plus/minus] end of the microtubule, while dynein heads for the [plus/minus] end.
Kinesin walks towards the plus end of the microtubule, while Dynein heads for the minus end.
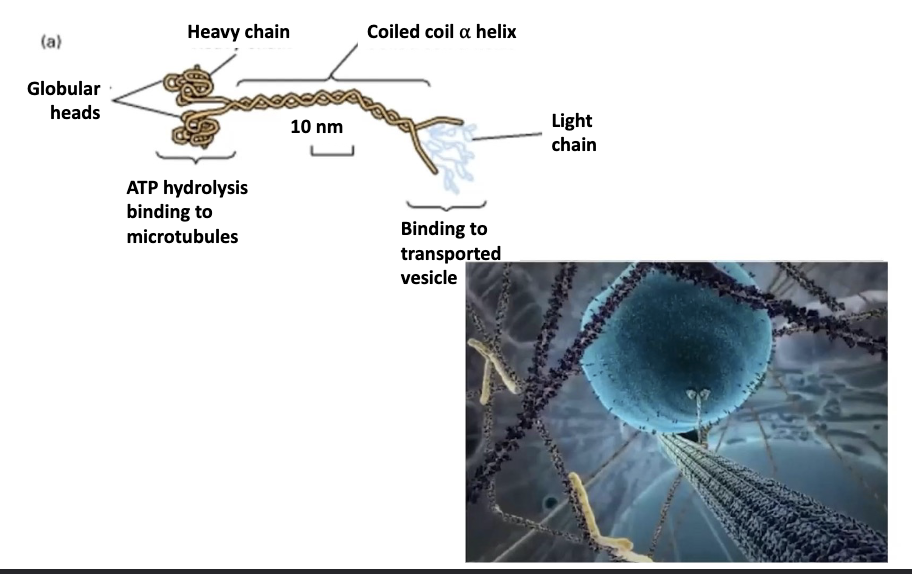
T/F: Kinesin has 2 feet (or “motor domains”) that walk along the microtubule. And a tail region that binds the cargo.
true!
Specifically, Kinesin has 2 light chains and 2 heavy chains. The globular heads at the N-termini of the heavy chains are called motor domains.
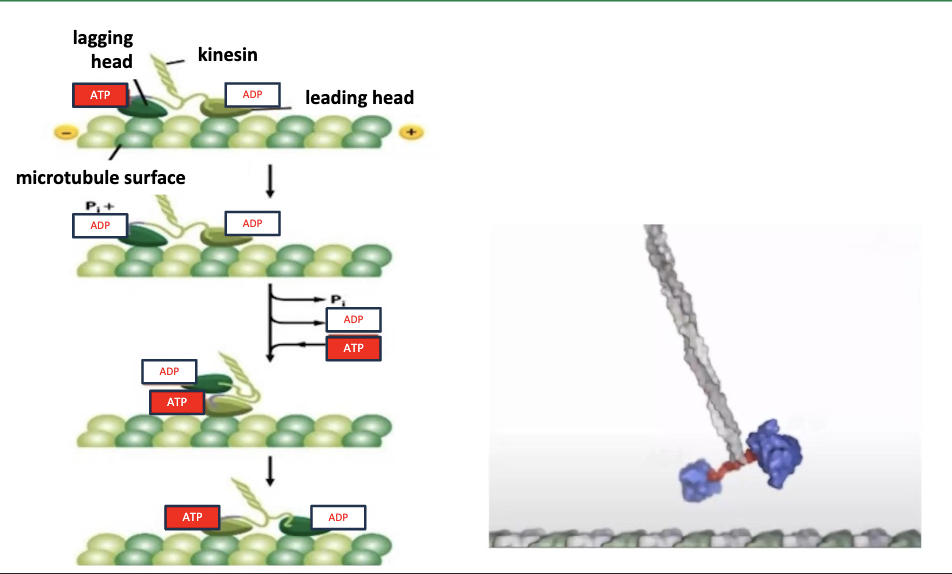
How does kinesin actually walk?
ATP powers it! the motor domains do this hand-over-head motion that is powered thanks to ATP causing conformational changes!
There are two motor domains in the kinesin dimer and one is always ….
… is ALWAYS attached to the microtubule. The two heads of kinesin are coordinated, so that at any one time they are present in complementary stage of the chemical cycle.
One head lags, the other head leads.
Hand-over-head motion refers to a leading head and lagging head. Tell me more abt how the chemical cycle works.
The lagging head (dark green) is bound to ATP and the leading head (light green) is bound to ADP.
ATP‐ bound kinesin has a higher affinity for the microtubule than ADP‐bound kinesin. In the lagging head, the ATPase motor head hydrolyzes ATP to ADP + Pi.
This reduces the affinity of the lagging head for the microtubule. In the leading head, ADP is exchanged for ATP, this increases the affinity of that leading head for the microtubule.
This binding of ATP (along with the hydrolysis of ATP in the lagging head), induces a conformational change in the neck region that causes the lagging head to swing forward in front of the leading head toward another microtubule binding site.
This resets the cycle back to the top!
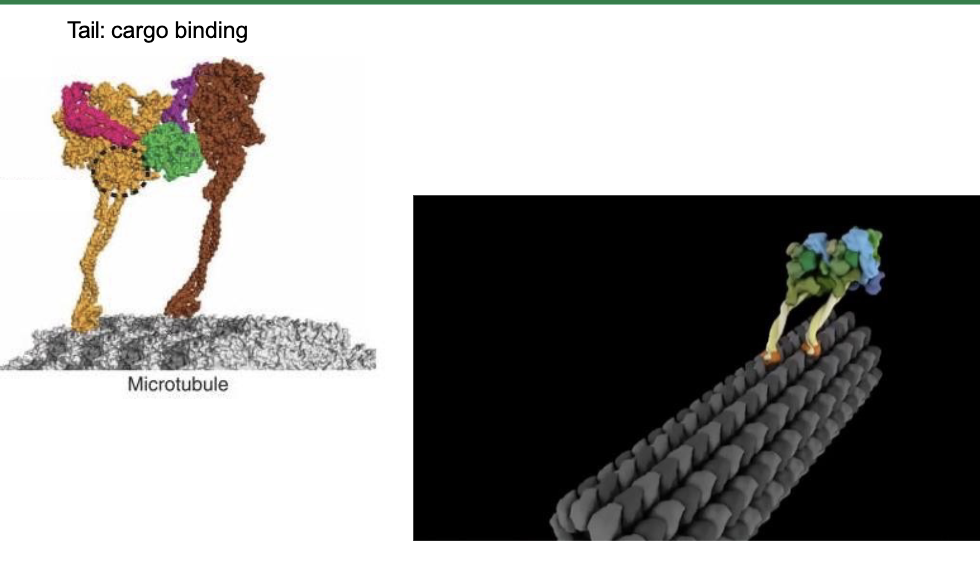
Dynein is [bigger/smaller] than kinesin.
Dynein is BIGGER, MUCH BIGGER, than kinesin.
Dynein moves AWAY from the cell periphery, i.e….
i.e. TOWARDS the MTOC of the cell!
What are the 2 forms of dynein?
Cytoplasmic dynein: associated with the microtubules and they direct movement of organelles and vesicles in the cytoplasm
Axonemal dynein: found in structures that power the movement of whole cells such as the cilia in some cells, or flagella in cells such as sperm cells.
What is the structure of dynein?
Dynein contains two identical heavy chains and a variety of intermediate and light chains.
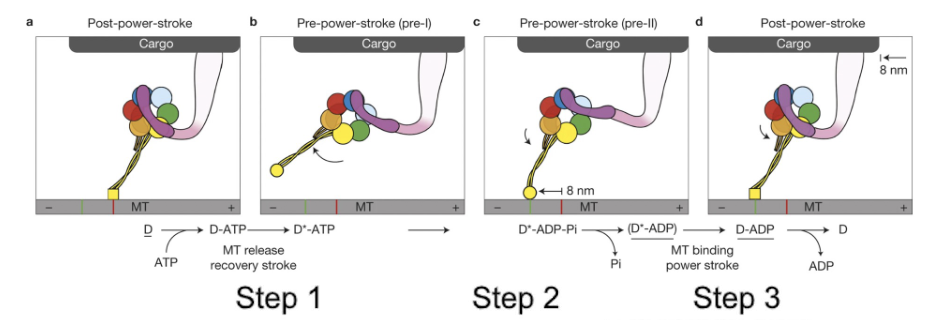
How does dynein walk along a microtubule?
-Each "leg" of dynein moves using ATP hydrolysis
-The ability of dynein to move cargo is driven by the power‐stroke of the linker (that is the purple arm shown here, near the cargo attachment site), that occurs with the release of inorganic phosphate from ADP.
-Within a dynein dimer, the dynein proteins alternate power‐strokes to move the cargo.
ATP binding releases the motor head group from the microtubule.
Then ATP hydrolysis, dynein‐ADP+Pi can now attach to the microtubule.
Then the release of the inorganic phosphase (Pi) powers the power‐stroke of the linker, which pulls cargo to the left here.
In the end, with each power stroke, the cargo moves towards the minus end by 8nm.
If both kinesin and dynene can attach to the same cargo, wouldn’t they pull in opposite directions. Like a cellular tug-o-war.
What can decide which wins the tug-o-war?
A buncha factors!
signals within the cell, the cargo’s locn, what type of cargo it is, even extracellular signals, etc.
T/F: So there can be bi-directional movement of vesicles.
true!
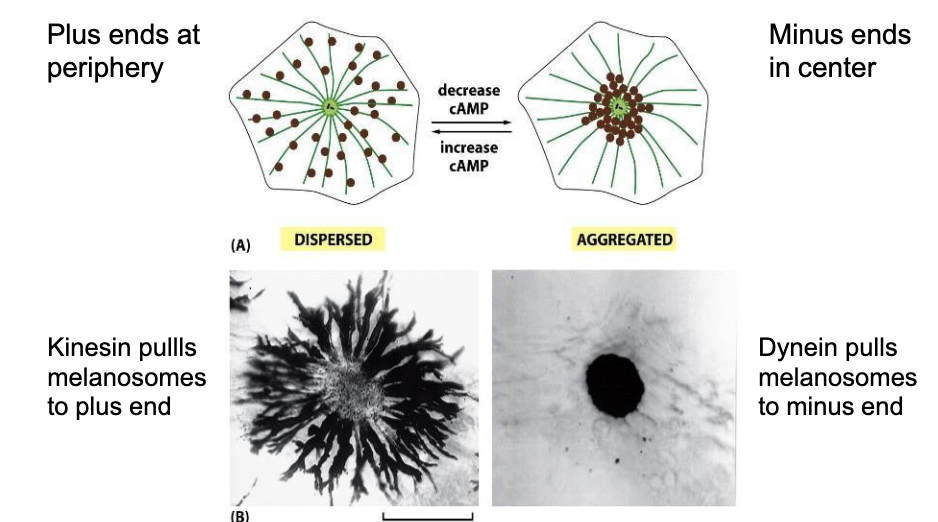
Walk me through how melanosomes get carried thru a cell in fish (fish skin cells in particular).
-When kinesin is active, it carries melanosomes towards the plus end of the microtubules out near the edge of the cell (making the cell and thus the fish look darker).
-When dynesin is active, it pulls those melanosomes back towards the minus ends of the microtubules (at the center of the cell) . So clustering them at the center of the cell make the fish look lighter.
The plus ends of microtubules are at the cell [centre/edge].
The minus ends of microtubules are at the cell [centre/edge].
The plus ends of microtubules are at the cell edge.
The minus ends of microtubules are at the cell centre.
Remember we discussed that when microtubulin transport gets messed up, it can have serious consequences for the cell. Like cancer.
How?
Cancer is just uncontrolled cell division. Microtubules form the mitotic spindle, which is essential for separating chromosomes during cell division.