Orgo 1+2 lab final exam
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
55 Terms
Four general notebook sections
Purpose statement, Safety and set-up, procedure, data / observation
What is written in the safety setup?
Compound hazards, comments
T/F: an unexcused absence from lab will result in a zero on the related safety and lab citizenship grade
T
T/F: The lowest post-lab assignment score of every student will be dropped
T
The people who will most likely read a specific piece of writing
Audience
The aims, goals or intentions of the writer
purpose
readers with expert-level knowledge in a specific area of chemsitry
expert audience
readers with significant scientific knowledge, but not in the specific area targeted in the written work
scientific audience
readers learning chemistry
student audience
Readers with little or no chemistry knowledges
general audience
To achieve conciseness, chemists use what that are nouns that are formed from other parts of speech, usually by adding such endings as -tion, -sion, -ment, -ity, -sis, -ence
Nominalizations
coveted skill among chemists, say only what needs to be said, deleting unnecessary words ( words that add little substance, state the obvious, or can be inferred by other words in the sentence)
Concisely
words that soften interpretations and suggest that interpretations are not absolute facts
Hedging words
two major purpose of a discussion are to and suggest
interpret results and broader implications of findings
to strengthen and support results or interpretations, using evidence from the literature
corroborate
the order in which you present findings (in results) and interpret findings (in discussion) should be
parallel
the word we (used to refer to the authors of the worK) is commonly used to achieve the following purpose in the ____ section
discussion
Which of the following best describes the purpose of IR spectroscopy?
To elucidate or confirm the identity of an unknown compound by identifying the functional group present
In an IR spectrum, the range of 4000-2700 cm-1 generally corresponds to which bonds?
Bonds to hydrogen
Which of the following is hazard associate with 4-aminophenol
organ damage through prolonged or repeated exposure
which of the following is hazard associated with benzophenone?
harmful to environment / aquatic life
Which of the following is a hazard associated with cyclohexane?
flammable
Which of the following is a hazard associated with 2-methyl-2-butanol?
flammable
T/F: The ATR crystal mount on the IR spectrometer must be cleaned before and after running each sample
T
As you wait to run your unknown samples, what other task will you be doing?
going through your drawer and checking all the glassware and equipment in it
What kinds of unknowns will you be analyzing in the first lab IR spectrum
one solids and one liquid
when you export your spectrum and save it to your USB drive, what do you save the file as?
.tsv
When does boiling occur?
1) when the energy gained by a liquid overcomes the intermolecular interactions in the liquid phase
2) When the total vapor pressure of the substance is equal to the atmospheric pressure
3) When the total vapor pressure of the substance is less than to the atmospheric pressure
1 and 2
what is the definition of vapor pressure?
The total pressure of a vapor in equilibriums with its liquid phase
Which of the following statements about miscible liquids is correct?
1) the components form a homogeneous solution
2) the partial pressure of each component is the vapor pressure of the component multiplied by the mole fraction of the component
3) each component exerts it own vapor pressure
1,2,3
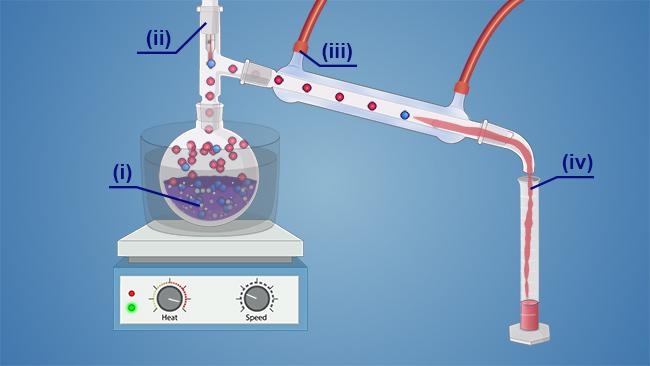
In the distillation setup shown, what is the distillate?
iv
When setting up distillation apparatus, which port on the condenser serves as the water inlet?
the port farthest from the round-bottom flask
How does the composition of the distillate change over the course of a distillation
it begins rich in the more volatile component, and ends rich in the less volatile component
cyclohexane and toluene have boiling points of 90.8 c and 110.6c , respectively, If distilling a mixture of these two compounds, which one will distill first?
cyclohexane
according to the sds, which of the following is a possible hazard for isopropanol?
flammable
What must be done when assembling the distillation apparatus to prevent the loss of vapor?
apply vacuum grease to all the joints
in a simple distillation setup, what is the sequence of equipment from the bench top to the round bottom flask?
Lab jack, stir plate, heating mantle
T/F: Solubility generally increase with increasing temperature
T
Which of the following is true regarding choosing a solvent for recrystalllization?
The desired solid must be completely soluble in a hot solvent
How much solvent should ideally be used for dissolving a crude mixture for recrystallization?
The minimal amount needed to completely dissolve the solids
Which of the following is/are part of a vacuum filtration apparatus?
1) FIlter flask
2) erlenmeyer flask
3) Buchner funnel
4) Filtration adapter
5) 10 ml graduated cylinder
1,3,4
You started with 5.00 g of crude mixture. After recrystallization, the mass of solid was 4.25g. What is the percent recovery?
85.0%
How will the purity of the recrystallized product be determined?
Melting point
when determining the melting point of a solid, the puree the material, the melting point is _____ and _______
narrower, close the literature value
according to the SDS, which of the following are possible hazards for n-bromosuccinimide (nbs)
corrosive and oxidizer
T/F: You should put your recrystallized NBS in the oven to dry it quickly
F
Where will you dispose of the aqueous filtrate waste at the end of experiment?
acid waste
Liquid-liquid extraction are based on which of the following properties?
solubility
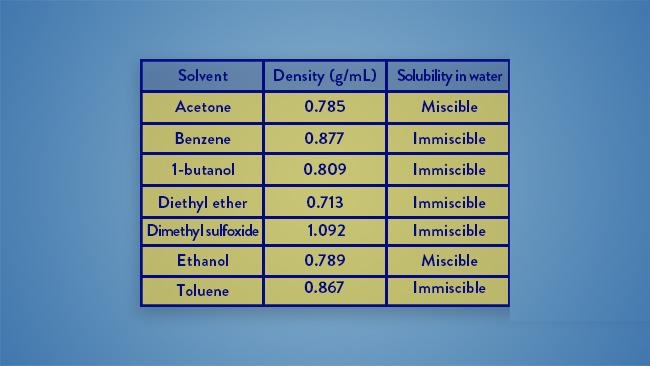
A student mixed their aqueous reaction mixture with acetone to perform a liquid-liquid extraction. After shaking the mixture in a separatory funnel and allowing the mixture to settle, which of the following outcomes is expected?
there are no layers present
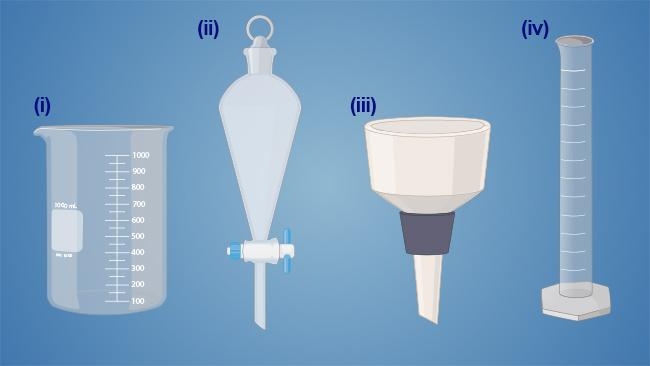
Which of the following glassware is most appropriate for performing liquid-liquid extraction?
2
A liquid-liquid extraction is conducted using the immiscible liquids dichloromethane (density=1.33 g/ml) and water (density = 1.00g/ml) Which of the following is true regarding this extraction?
The dichloromethane will comprise the bottom layer because it is denser than water.
The dichloromethane will comprise the aqueous layer.
The dichloromethane will comprise the top layer because the aqueous layer is always on the bottom.
The dichloromethane and the water will not form layers
the dichloromethane will comprise the bottom layer because it is denser than water
Why is it important to vent the separatory funnel frequently during mixing?
Venting releases the build-up of vapor pressure caused by agitation of the organic solvent.
Gaseous products formed by the reaction of the two solvents would build up if not released.
Venting releases trapped air bubbles in the solution that would otherwise disrupt the separation of the two phases.
Oxygen is required for the successful separation of phases.
Venting releases the build-up of vapor pressure cause by agitation of the organic solvent
You need to isolate a basic organic compound from a mixture of nonbasic compounds
this can be achieved by adding (acid or base), which will allow the resulting molecule to partition into the (organic layer or aqueous layer)
acid, aqueous layer
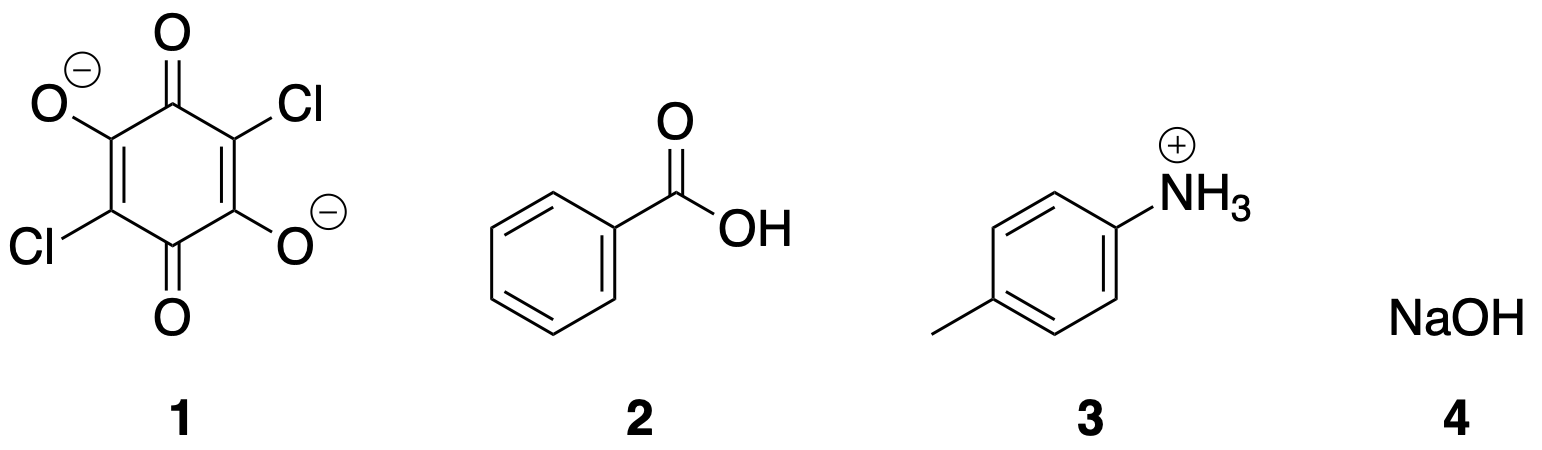
Into which phase (aqueous or organic) would you expect each of the following compound to partition?
Compound 1,2,3,4,
aqueous or organic
Compound 1,3,4 are aqueous
Compound 2 is organic
According to the sds, which of the following is a hazard of diethyl ether?
flammable
corrosive
radioactive
oxidize
flammable