What are alcohols?
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
What is the general formula for alcohols?
CnH2n+1OH
Alcohols are formed when…
halogenoalkanes undergo nucleophilic substitution reactions with aqueous hydroxides
We call a molecule an alcohol if it’s an alkane where a hydrogen is replaced by a(n)...
OH/hydroxyl group
What’s the name of this molecule?
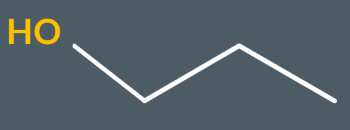
propan-1-ol
How we classify alcohols depends on the number of...
carbons bonded to the C-OH carbon.
Is this alcohol primary, secondary or tertiary?
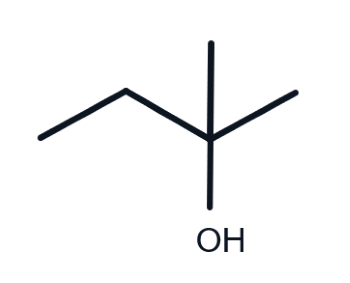
tertiary
is this alcohol primary, secondary or tertiary?
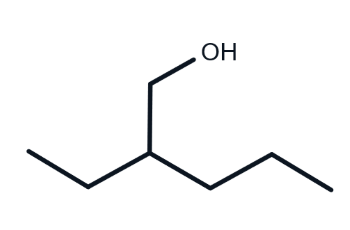
primary
What’s the name of this molecule?
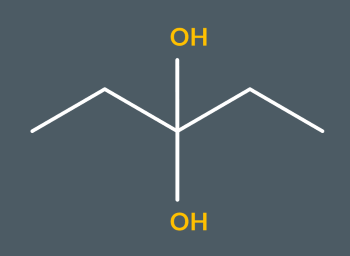
pentane-3,3-diol
What’s the name of this molecule?
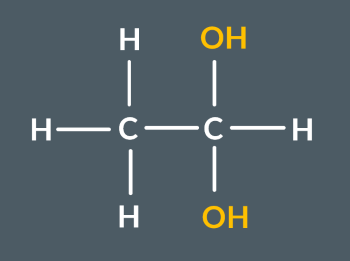
ethane-1,1-diol
What’s the name of this molecule?
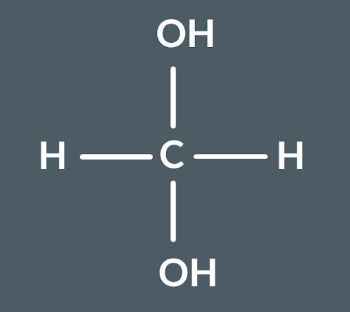
methanediol
diol
an alkane where 2 hydrogen(s) are replaced by hydroxyl groups.
What’s the name of this molecule?
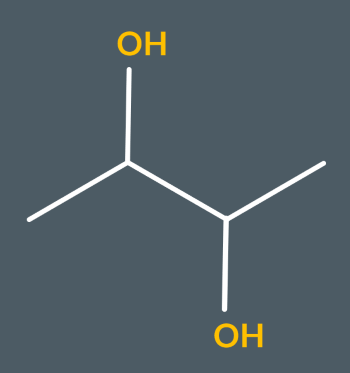
butane-2,3-diol
The strongest intermolecular force between alcohol molecules is…
hydrogen bonding
volatillity
how easily a liquid evaporates
Because oxygen is more electronegative than hydrogen, we say that the oxygen-hydrogen bond is....
polar
Are alcohols soluble in water?
highly soluble in water
In an alcohol Solubility decreases as the length of the carbon chain increases because…
nonpolar substances are highly insoluble in polar substances.
a greater region of the molecule is nonpolar.
why is methanol more soluble in water than butanol?
Butanol has a longer carbon chain than methanol
A smaller region of methanol is nonpolar
Predict whether ethanol or ethane-1,2-diol is more soluble in water.
Ethane-1,2-diol
has one more OH group than ethanol
This means that ethane-1,2-diol can form more hydrogen bonds with water molecules