Series and Bohr Model
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
What is the Rydberg equation used for?
It describes the wavelengths of visible lines in the emission spectrum of hydrogen (and other elements).
What is the Rydberg equation formula?
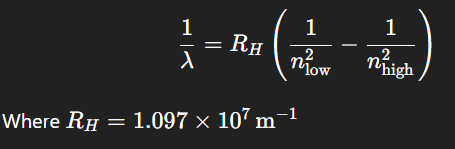
What is the Lyman series in the hydrogen spectrum?
A series where the final energy level is n1=1, it lies in the ultraviolet region.
What is the Paschen (Bohr) series in the hydrogen spectrum?
A series where the final energy level is n1=3, it lies in the infrared region.
What is the ground state of an atom?
The most stable, lowest energy arrangement of electrons in an element or compound.
What is the excited state of an atom?
Any arrangement of electrons with energy higher than the ground state.
What is decay in atomic transitions?
When an atom in an excited state returns to the ground state by emitting a photon. The photon’s energy equals the difference between the two energy states.
How is the energy difference between atomic levels calculated?
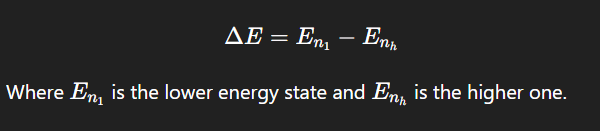
What is emitted when an electron transitions to a lower energy level?
Light, but only of specific wavelengths (a line spectrum).
What happens when an atom emits or absorbs light?
Emits: The atom decays to a lower energy state.
Absorbs: The atom becomes excited to a higher energy state.