Reservoir MT
1/93
Earn XP
Description and Tags
Modules 1+2+3+4+5(theory)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
94 Terms
Is IGIP stated at reservoir or standard conditions?
Standard
IGIP → the total volume of gas in the reservoir at standard conditions.
Metric Units: se6m3 (million standard cubic metres)
What are the 3 methods of estimating IGIP? Rank them from least accurate to most
Volumetrics - least accurate, assumes an average net pay
Planimetrics - more accurate than volumetrics because BV and PV is calculated more accurately (using a contour map)
Material Balance (P/z method) - most accurate because it is based on the actual production history of the reservoir.
When using Material Balance to estimate IGIP - ie. using P/z vs. Cum. Production - what are the 4 necessary assumptions?
Closed tank reservoir
No water influx
Little or no water production
Homogeneous reservoir
What are the units of the P/z slope?
kPaa/se6m3

At what conditions is Recoverable Gas stated?
Standard
Recoverable gas → the total volume of gas that can be recovered or produced over the life of the well.
Metric Units: se6m3
Imperial Units: MMSCF
What is a typical recovery factor range for gas reservoirs?
60% to 95%
Gas condensate reservoir → 60% to 70%
Dry gas reservoir → 90% to 95%
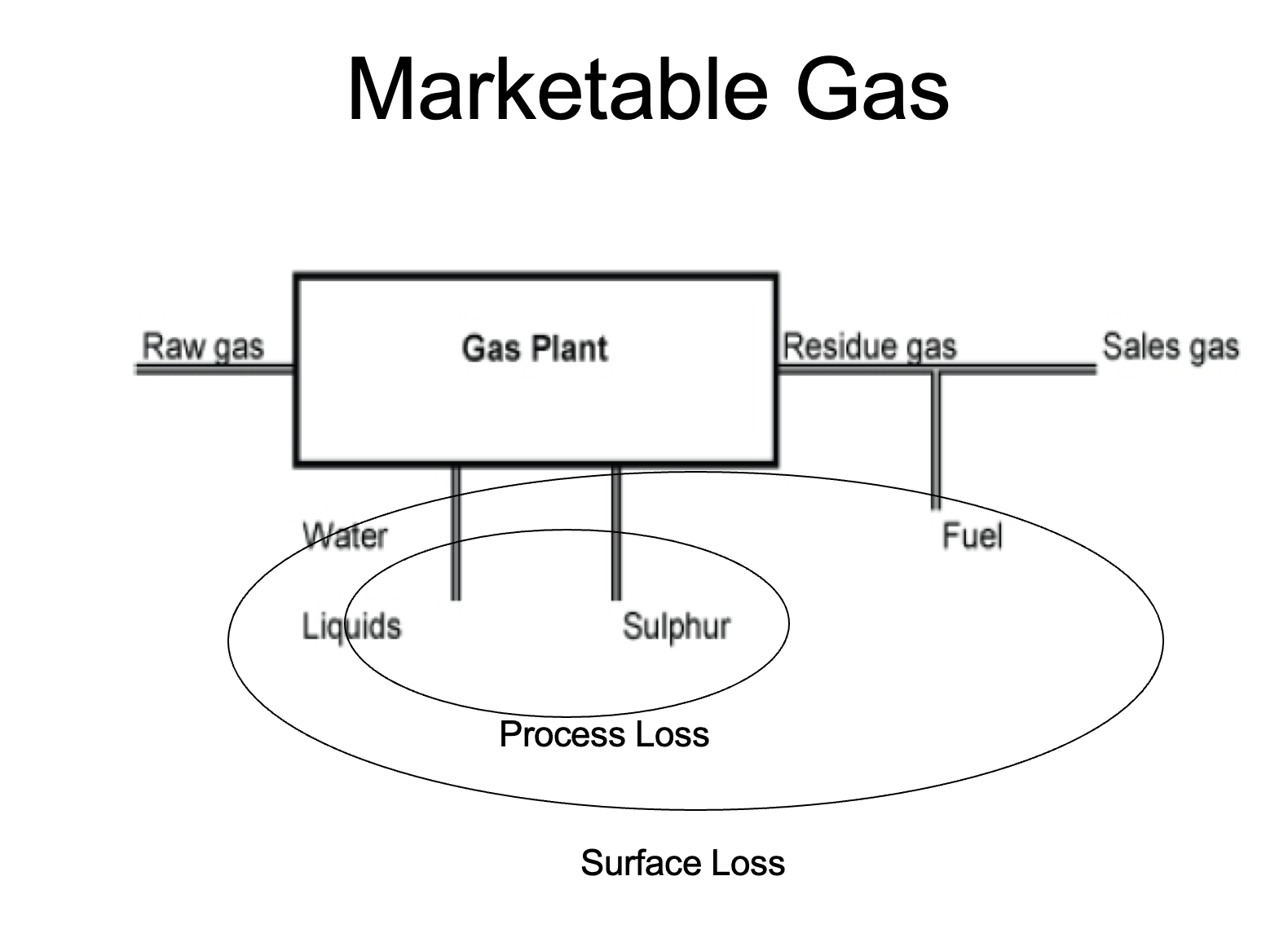
Explain the difference between Recoverable Gas and Marketable Gas
Marketable Gas is the total volume of gas that can be sold to market at market specs.
Therefore, it will always be less than Recoverable Gas, as some processing is required to bring the raw produced gas to sales specs, and all processing facilities have some process loss and require some fuel to operate
What is residue gas?
Residue gas is the volume of gas after processing (and ususally before F&M is taken off - but not always!)
While sales gas or marketable gas is the gas after processing (residue gas) minus any F&M (ie. minus all surface losses).
In a gas condensate reservoir, gas-cycling is often used to improve recovery, wherein lean gas is injected and wet gas-condensate is produced. The process of injection is continued until most of the gas-condensate is recovered, or the liquid content is too low to economically re-inject the lean gas. At that point, injection is terminated and the reservoir is allowed to produce below the dew point until abandonment. The reservoir would now be stated to be in _____-______ mode.
blow-down
no more injection = reservoir is producing solely off its own pressure = reservoir pressure is depleting = reservoir is “blowing down”
Define
Condensate Yield Ratio
NGL Yield Ratio
Sulphur Yield Ratio
These are all ratios of the inlet, or raw gas rate.
Condensate yield ratio: ratio of condensate (ie. liquid) produced to the raw inlet gas
Unit sm3/se3m3
NGL yield ratio: ratio of NGLs (ie. liquids) produced to the raw inlet gas
Unit sm3/se3m3
Sulphur yield ratio: ratio of sulphur produced (solid) to the raw inlet gas
Unit mT/se3m3
NOTE: these are all based on the raw inlet gas; they are never based on residue or sales gas.
What is the operating pressure of a gas cycling scheme?
At or slightly above the dew point
the whole point of re-injection is to keep the reservoir P above the dew point in order to produce more liquids
Below dew point = blow-down
In calculating SL, PL and F&M should be a fraction of what?
Raw gas
NOTE: F&M is sometimes given as a fraction of residue gas - in which case it must be converted to a fraction of raw gas before being combined with PL to get SL.
F&M as frac of raw = (Residue gas as frac of raw) x (F&M as frac of residue)
ie. a simple unit cancellation
In a gas cycling scheme, _____ gas is injected and _____ gas is produced.
lean injected and wet produced
From which gas stream is the heating value calculated?
From the residue gas stream only (ie. after processing)
In gas analysis, what is the primary goal of recombination?
To reconstruct the original composition of the gas as it exists in the reservoir
After liquids are extracted, will the the heating value of the residual gas stream be higher or lower?
Lower
more carbons = higher heating value
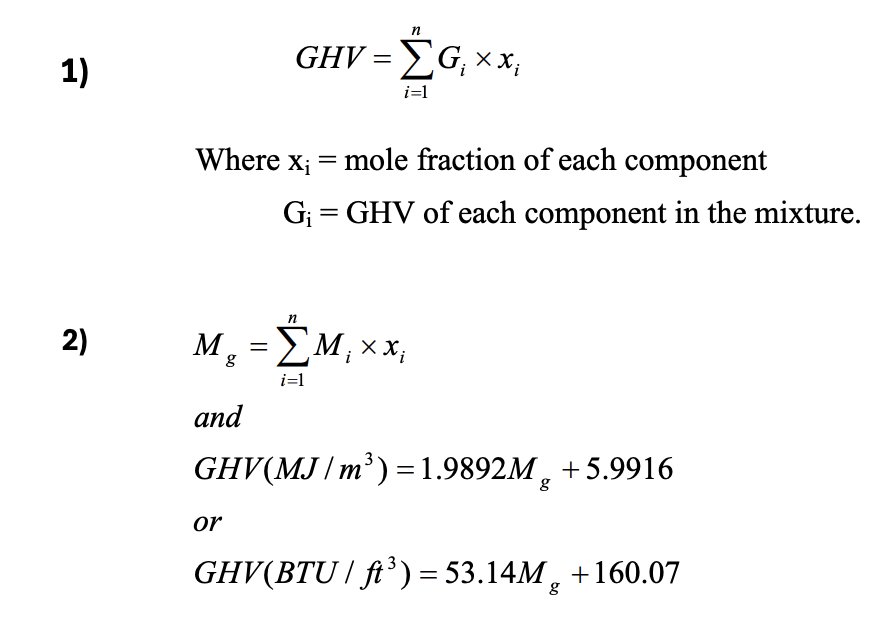
The gross heating value of a gas is a direct function of what?
The molecular weight of the gas
see formula sheet:
GHV (MJ/m3 ) = 1.9892Mg + 5.9916
What are the 2 methods of calculating the GHV of a gas?
Both require you to have the mole fractions of each component
mole frac. of comp. x GHV of comp. (given)
sum all comp. GHVs to get GHV of gas
mole frac. of comp. x MW of comp.
sum all comp. MWs to get MW of gas
plug into formula for GHV on formula sheet
A question asks you to combine a liquid and a gas stream, while giving the flow rates of each stream as molar flow rates. What conversion has to be done in order to combine the gas & liquid streams?
No conversion
molar flow rates can simply be combined because the units are the same
qtotal = qgas(molar) + qliquid(molar)
What is a GEL flowrate?
Gas-equivalent liquid flow rate → allows you to take a liquid flow rate (sm3/d) and convert it into a gas-equivalent flow rate (se6m3/d) so that it can be combined with another gas stream.
We cannot add 120.00 se3m3/d of gas and 3.00 sm3/d of liquid. Why?
a) because the units are different
b) because the streams are in different phases
b) because the streams are in different phases
volumetric flow rates depend on density, and density differs by phase
Note that molar flow rates are simply moles/unit time and therefore independent of density/phase - and so can be combined
Give the formulas for liquid and gas component recovery
where n = component (C1, C2, C6, etc.)
n gas recovery = n qgas / n qgas + n qGEL
n liquid recovery = n qGEL / n qGEL + n qgas
Be careful with production units
Example: You’re given values of average annual production in MMSCF/d and then asked to calculate the cumulative production over 10 years in BSCF
[(sum 10 yr daily averages in MMSCF)*365]/1000 = 10 yr cum. BSCF
A gas well in Alberta produces raw wet and sour gas at a flowrate of 38.26 MMSCF/d. The gas is processed at a nearby gas plant with process loss of 18%, and fuel & metering error of 8% of raw gas.
Draw the flow schematic for this question
Main thing to recognize is that the F&M comes off of the raw gas because it is stated in the question as a fraction of raw
∴ your residue gas & sales gas values will be off if you don’t clock this
See HW1 Q2

If you have the molar composition of the raw gas and then calculate the molar compositions of the liquid and gas streams → how can you quickly find the GEL flowrate if you know the raw gas flowrate?
qGEL = (sum of the moles in the liquid)*(qraw gas)
this works b/c the sum of the liquid moles is essentially the fraction of raw gas that condensed into liquid, assuming your raw gas composition was given for 1 mole of raw gas
What is another term for Expected Ultimate Recoverable (EUR) ?
Total Reserves
What is the GEL ratio?
GEL ratio = GEL rate/liquid rate
What is boundary dominated flow?
Boundary-dominated flow occurs when the pressure disturbance from production has reached the physical limits of the reservoir
in other words: the reservoir has produced long enough for the initial pressure pulse to reach the outer boundary of the reservoir
It is one of the assumptions used in Decline Curve Analysis
What is another term for the nominal decline rate, a?
what is its unit?
Instantaneous decline rate
Unit: 1/time (ie. 1/day 1/month 1/year etc.)
a = nominal decline rate = negative of the instantaneous slope of the exponential decline curve
‘instantaneous slope’ meaning the slope of the tangent to the curve at any one point

What are the 2 types of diagnostic plots used in decline curve analysis?
Rate-Time
Rate-Cum. (Rate vs. Cumulative Production)
What are the assumptions used in Arps’ Equations?
boundary dominated flow
tank depletion model
constant number of wells
constant permeability and porosity
constant separator and tubing conditions
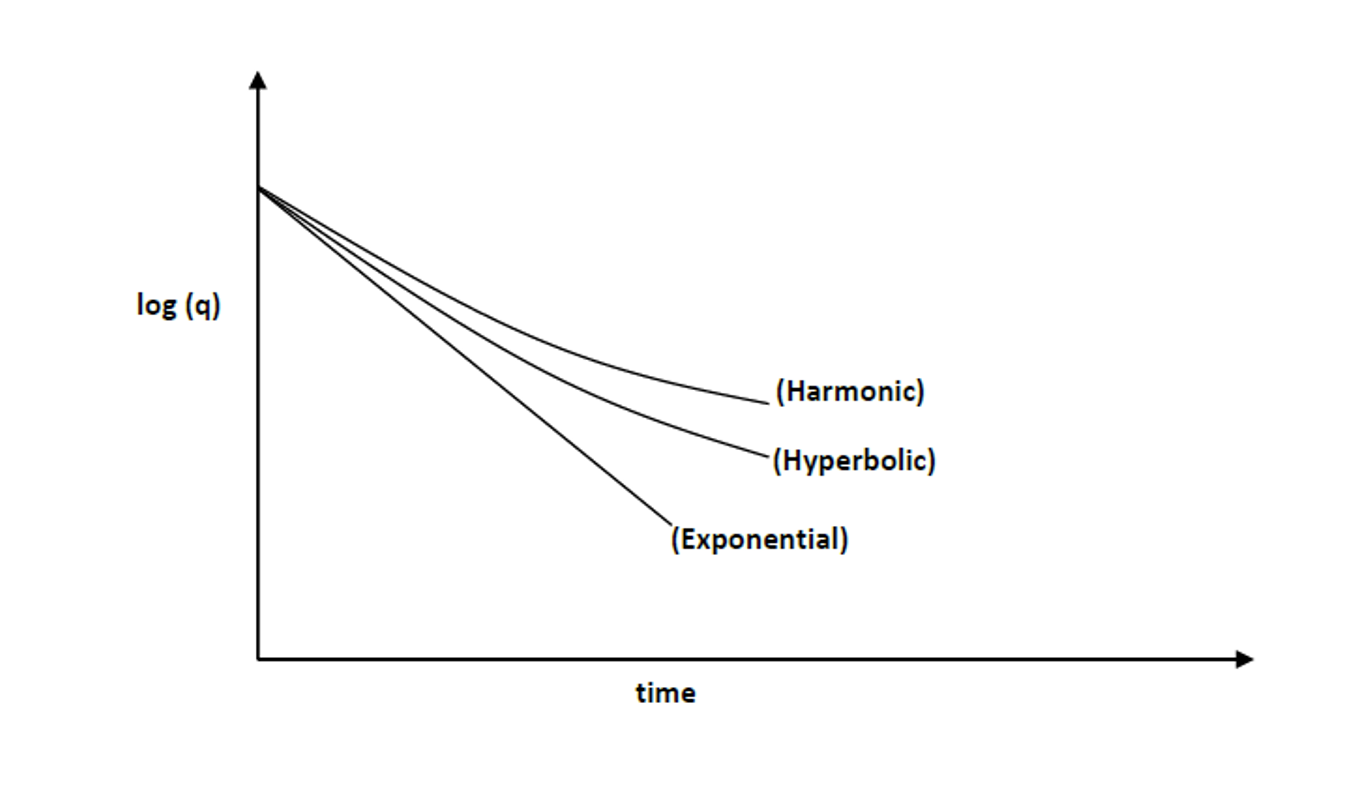
Which type of decline model has a constant nominal decline rate?
Exponential
A linear decline curve for rate vs. time on a semi-log plot indicates which type of decline model?
Exponential
can be seen from formula sheet: the rate vs. time equations with log/ln in them for exponential decline are both equations of a straight line (ie. in the form y=mx+b):
log(q) = -at/2.303 + log(qi)
ln(q) = -at + ln(qi)
For an exponential decline, plotting Rate vs. Cumulative Production on a Cartesian plot will result in what type of trend?
Linear (ie. straight line)
can be seen from formula sheet
Of the 3 decline models, which estimates the most pessimistic (minimum) value of the total reserve (EUR)?
Exponential
The cumulative production corresponding to the flow rate at abandonment, is know as what?
Expected Ultimate Recoverable (EUR)
Np @ qa
Gp @ qa
The percentage in change in the flow rate over a period of time (usually 1 year)
Effective decline rate, d
Unit: %/year
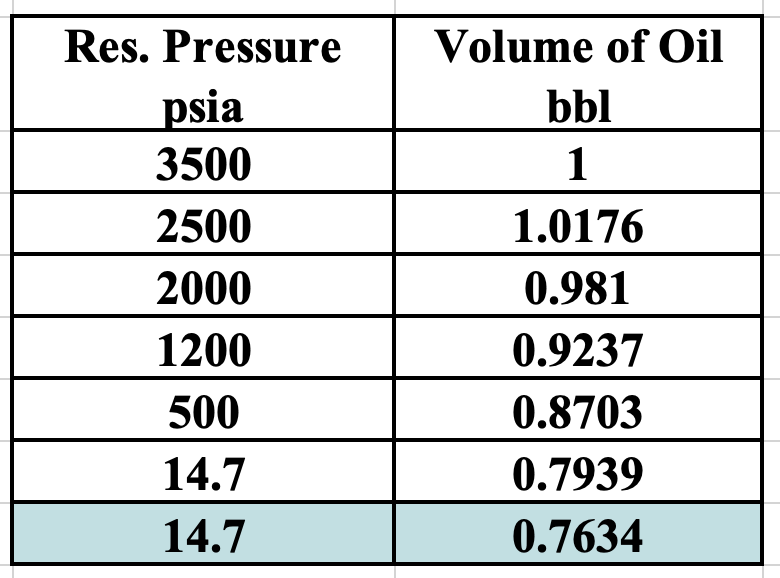
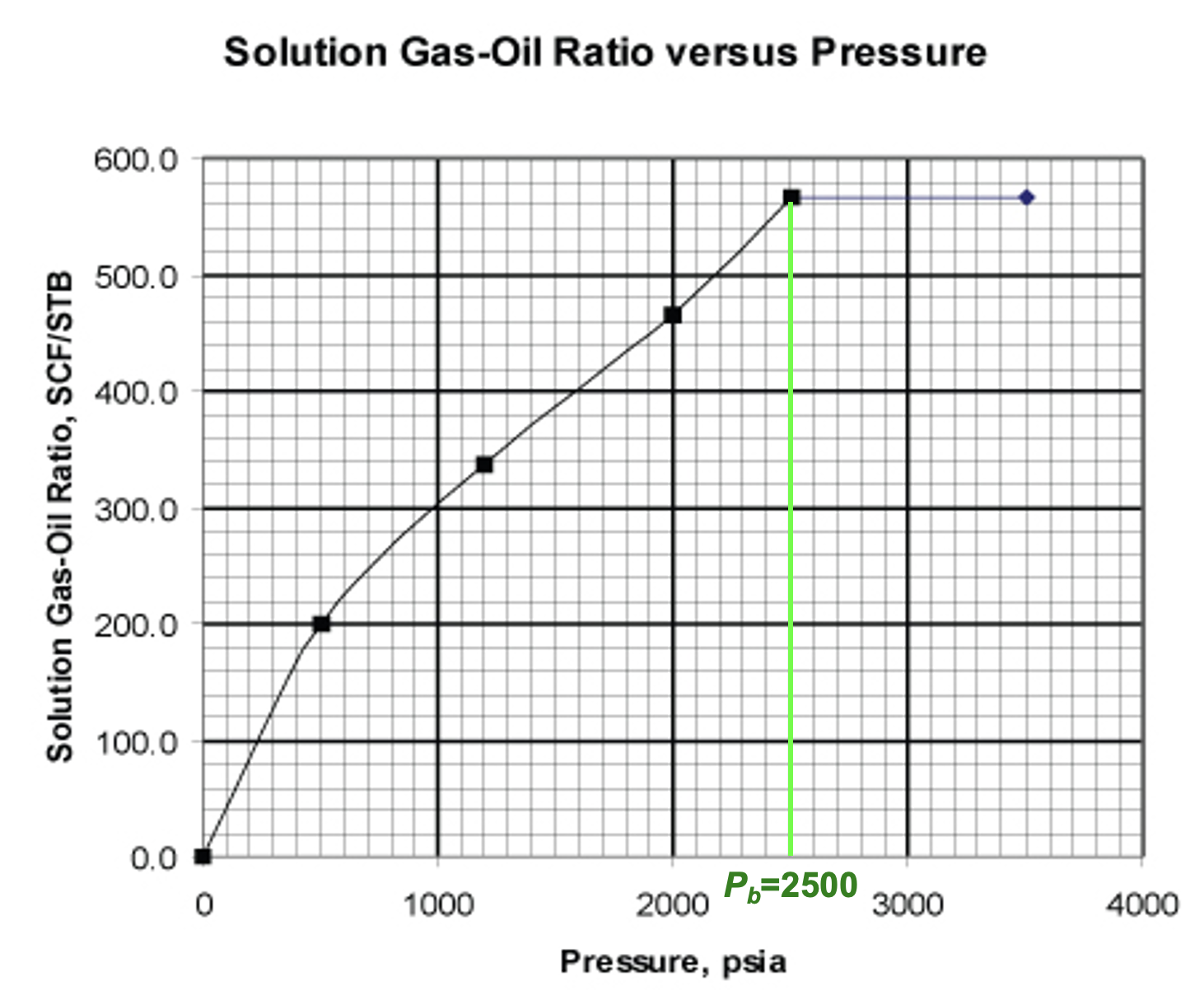
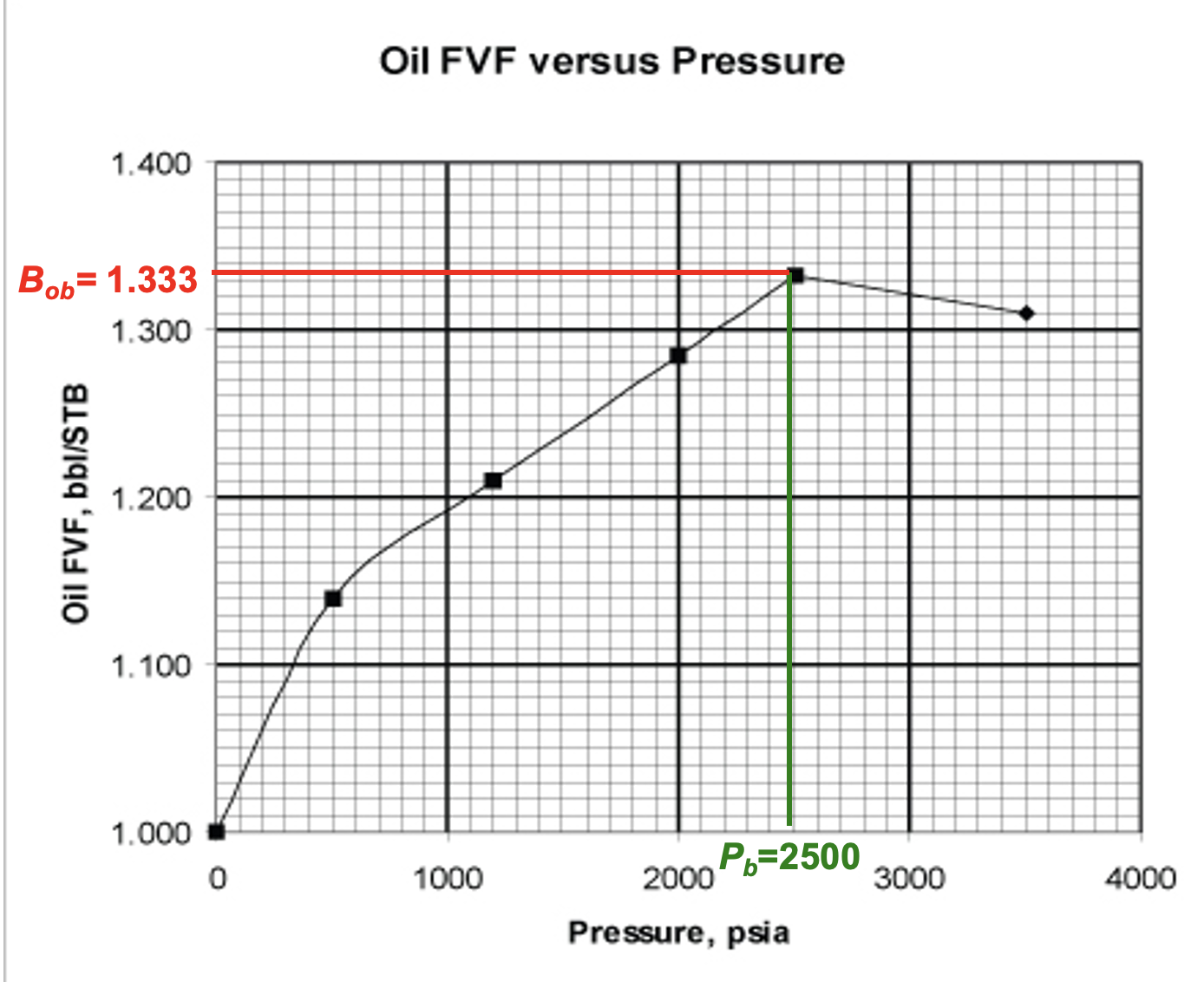
What is the bubble point?
2500 psia
max live oil volume
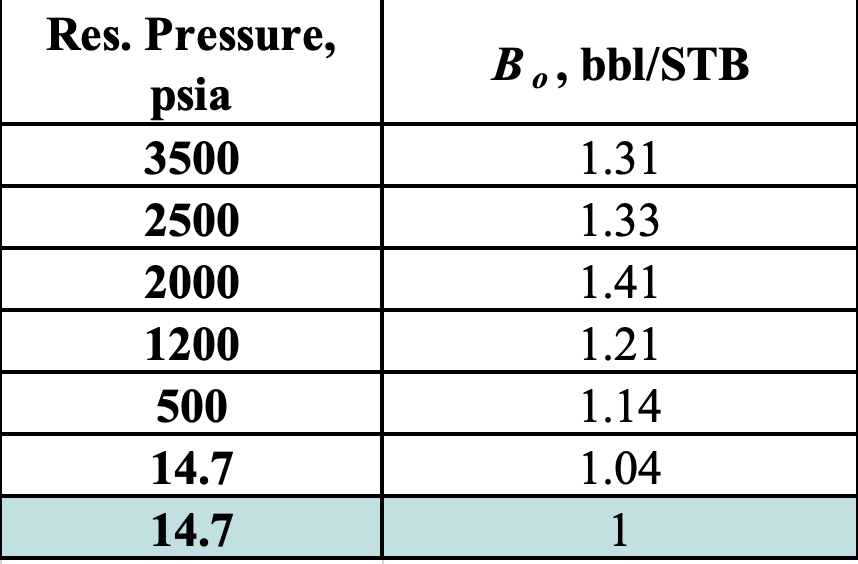
What is the bubble point?
2000 psia
max Bo
If the decline exponent, b, is greater than 1, what does this indicate about the reservoir?
b > 1 → reservoir is likely unconventional (assuming boundary dominated flow)
A family of curves as “pre-solutions” that are plotted for different values of b & ai
Type curves
What are the assumptions for IGIP and IOIP formulas?
IGIP
only gas + water (ie. no oil)
IOIP
only oil + water (no gas)
Which decline model is the most general form of decline analysis?
Hyperbolic
exponential and harmonic are considered ‘special cases’ of hyperbolic where b = 0 and b = 1 respectively.
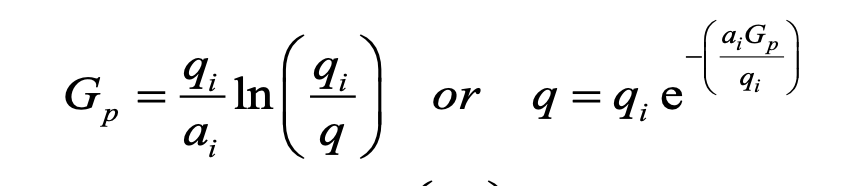
What is the unit of ai in this hyperbolic DCA equation?
1/day
b/c the q (rate) value is always in volume/day
In the Cullender & Smith method, which will always be larger?
a) the Pb estimated using the Trapezoid Rule
b) the Pb calculated using Simpson’s Rule
a) the Pb estimated using the Trapezoid Rule
This will always be an overestimation of Pb, given that, mathematically, the trapezoid method will always over estimate the area under a curve
Simpson’s rule then corrects this overestimation
For the majority of conventional reservoirs with boundary dominated flow, what range will the decline exponent “b” fall into?
0 to 1
Name 3 possible reasons for observing an “unusual” (ie. outside the 0 to 1 range) decline exponent “b”
1) Error in the analysis of the data where a b<1 can also be applied to match the historical production data.
2) The flow has not reached the boundary dominated condition yet and it is still transient flow.
3) Unconventional oil and gas reservoirs (such as tight oil, tight gas, shale oil, shale gas reservoirs) often exhibit b>1.
TorF: Decline analysis of unconventional reservoirs is different than traditional methods and requires its own set of modifications of the equations
True
Of the 3 decline models (exponential, hyperbolic, harmonic), which one has no straight line relationship?
Hyperbolic
evident from equations on formula sheet
In a gas material balance question, you are asked to calculate the recoverable raw gas at abandonment conditions - what are the 4 steps to this calculation?
a) Calculate the Pi/zi
b) Calculate the slope, m (rearrange the IGIP formula)
c) Calculate Pa/za
d) Calculate the RG @ abandonment
See Tutorial 1 Q1
(Ali mentioned that on a test, the Q may not be broken down into steps like the tutorial - so you should know how to get to RG w/o it being spelled out)
Convert 1 month to days
365/12 = 30.43 days/mo
Harmonic decline is a special case of ________ decline.
hyperbolic
(so is exponential)
You plot rate vs. cum. on a semi-log plot and obtain a straight line - which decline model is it?
Harmonic
look at the equations
The equation of the trend line (in exponential format) for Rate (sm3/d) vs. Cum. on a semi-log plot is y = 160e-0.0433x , what is the initial rate?
160 sm3/d
from formula sheet
The pressure profile plot (pressure vs. depth) is usually:
a) linear
b) non-linear
b) non-linear
The temperature profile plot (temperature vs. depth) is usually:
a) linear
b) non-linear
a) linear
TorF: The gas pressure gradient in a gas well is usually constant
False
gas is compressible
Gas density decreases with decreasing pressure → less weight per unit height → smaller incremental pressure change at shallower depth → non-linear curve
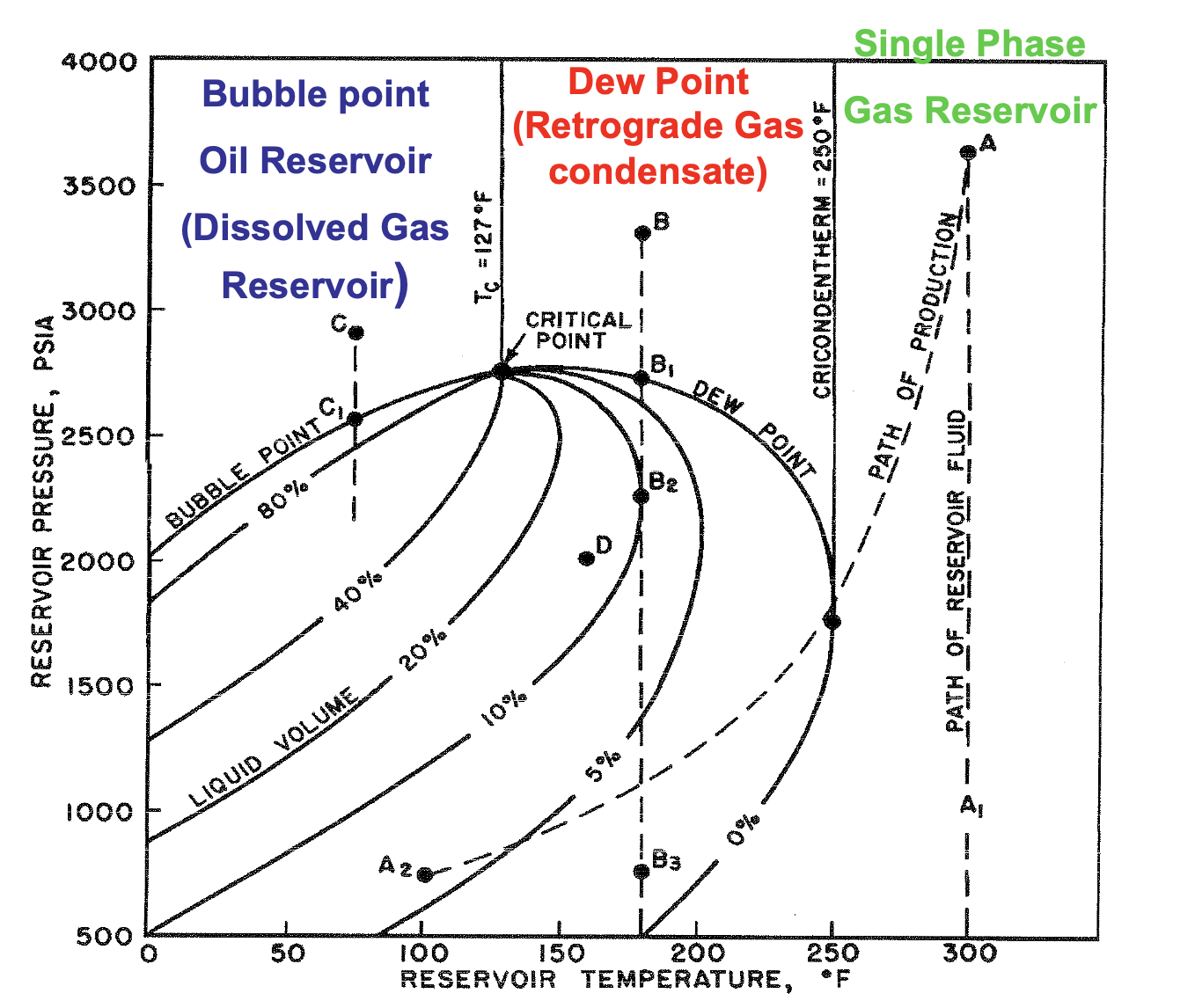
If T > Tc , what type of reservoir is it?
Gas
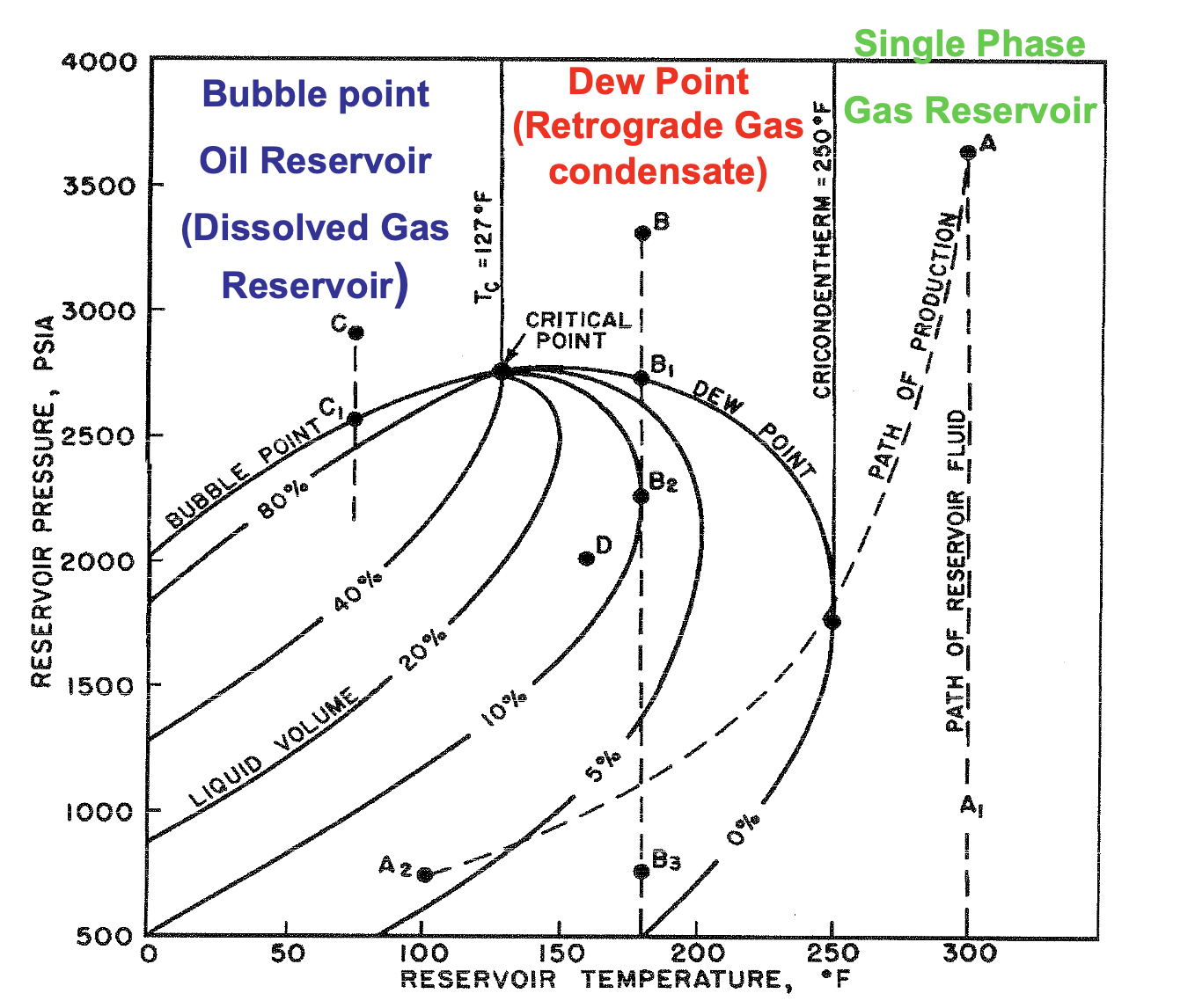
If T < Tc , what type of reservoir is it?
Oil
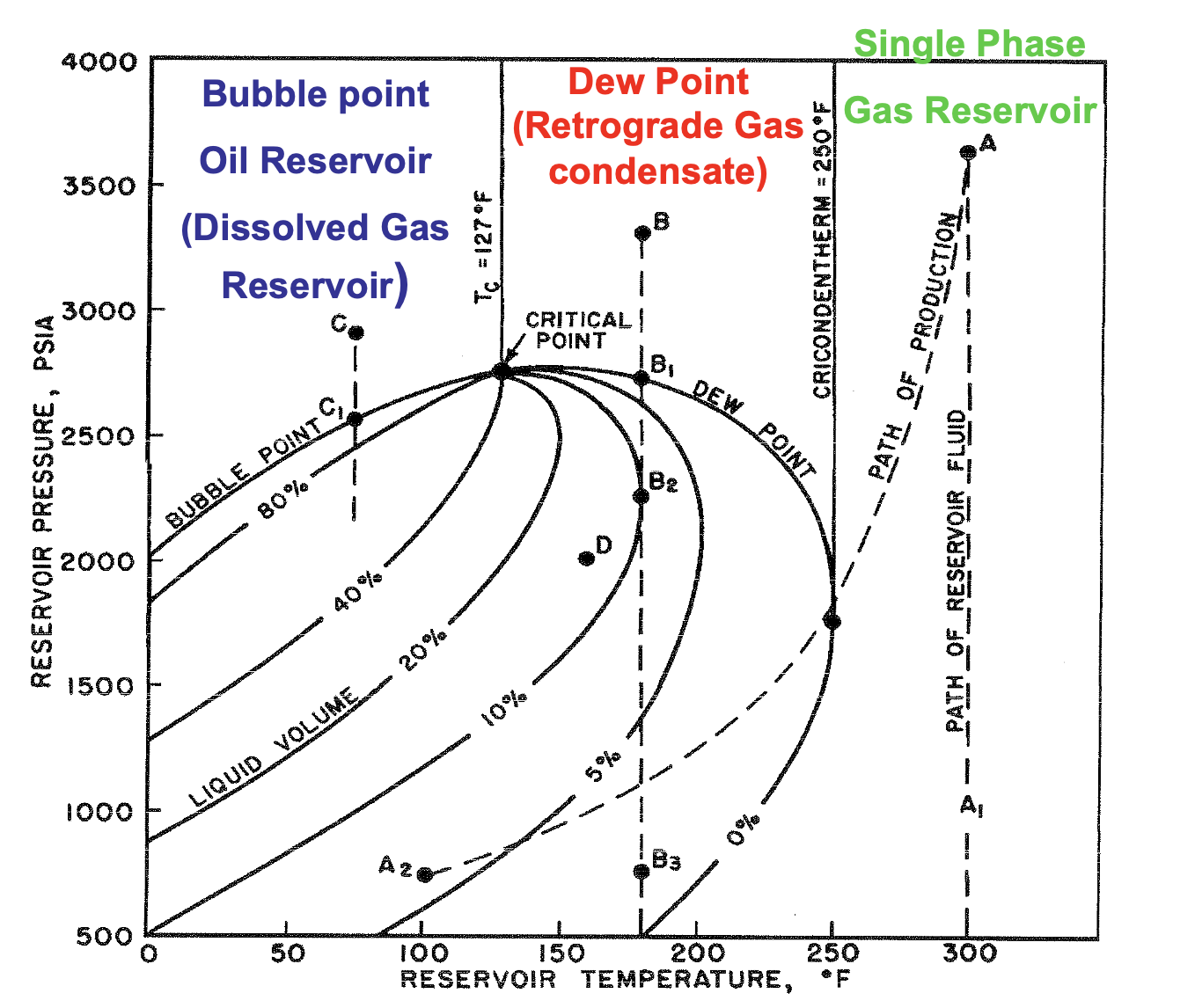
If Tc > T < Tcr , what type of reservoir is it?
Gas-Condensate
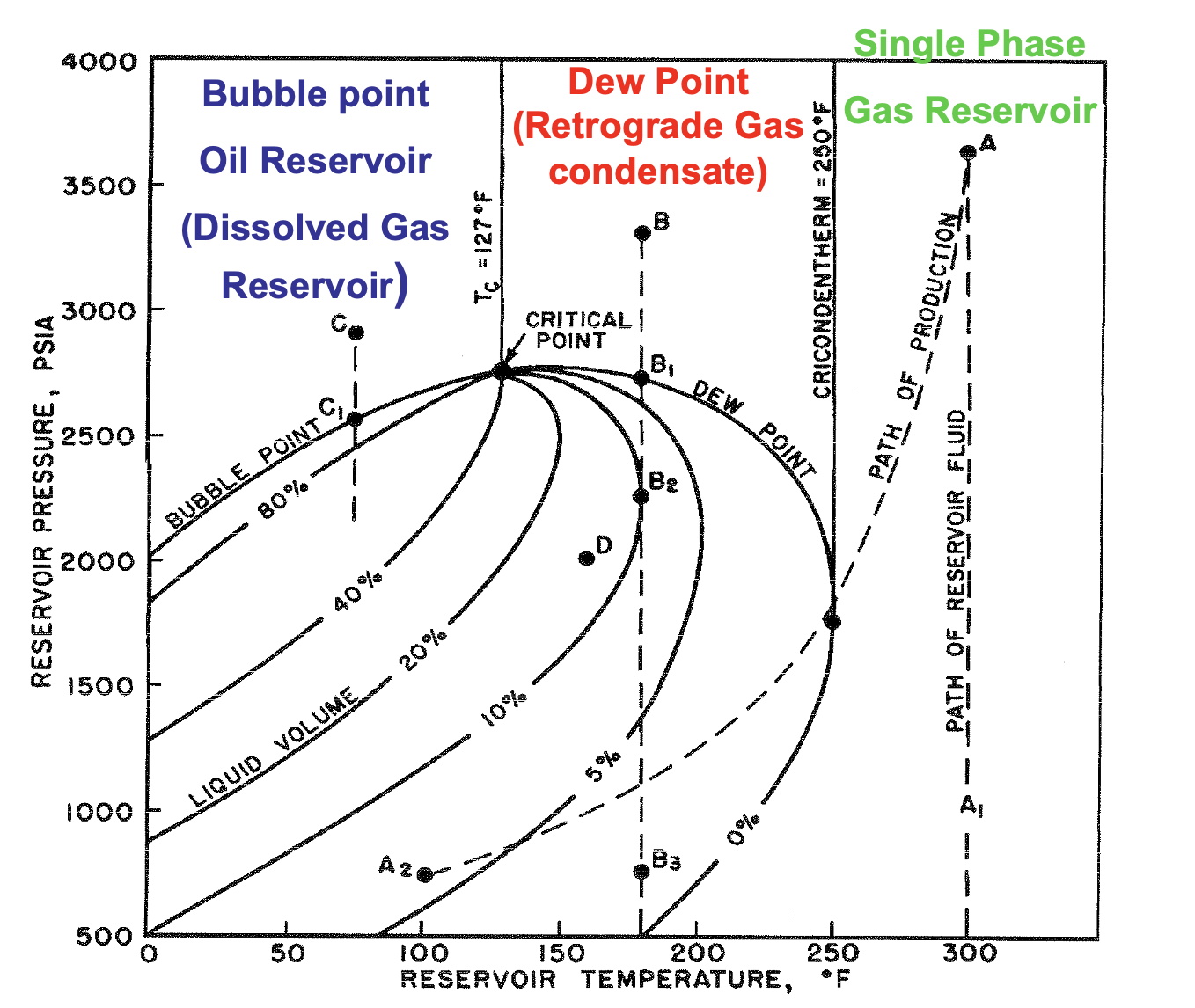
If T > Tcr , what type of reservoir is it?
Dry Gas
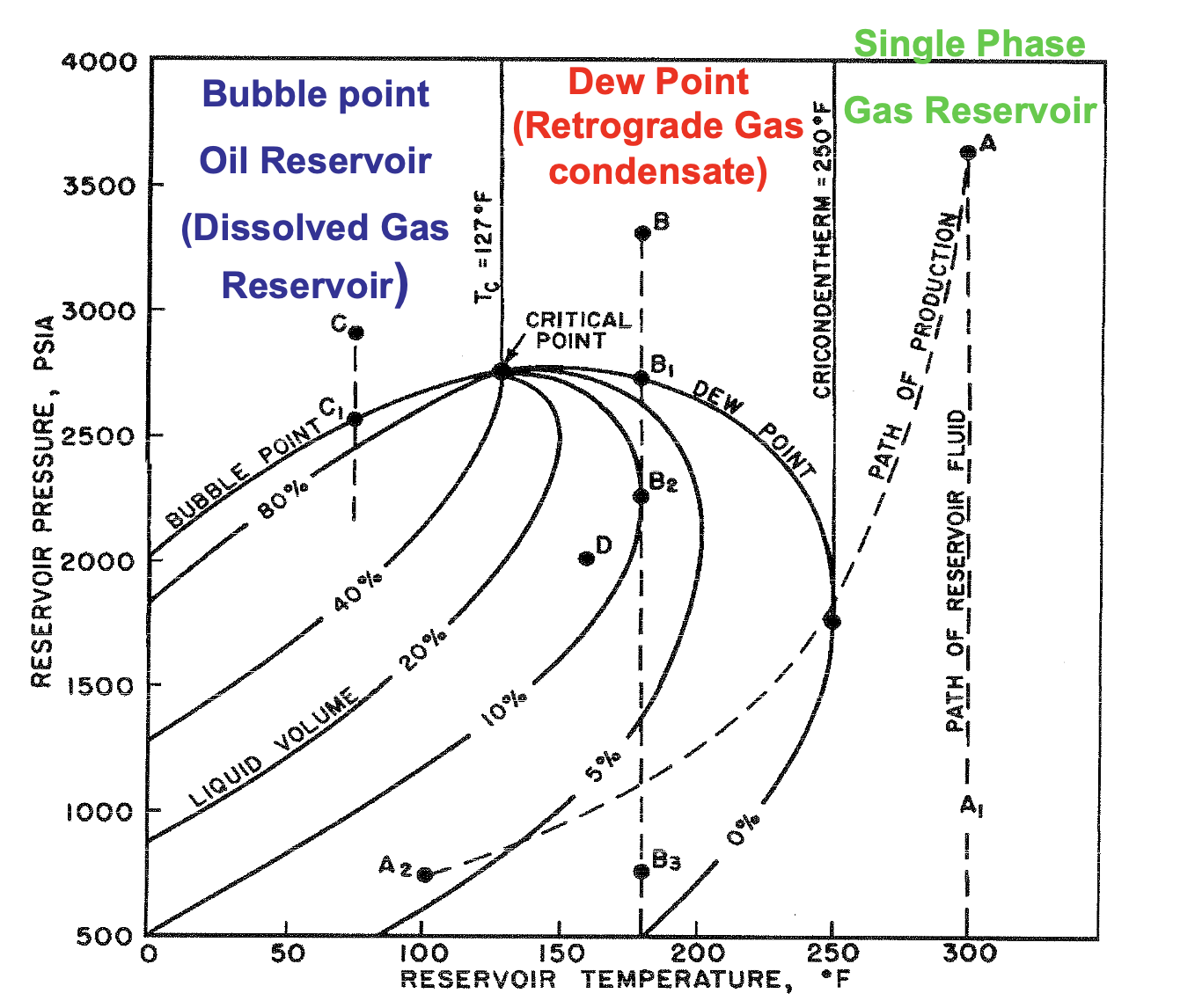
The maximum temperature at which liquid and vapor phases can coexist in equilibrium for a given hydrocarbon mixture
Cricondenterm
∴ at temps above the cricondentherm, only single phase dry gas is present
As gas pressure increases, density _____________.
increases
When temperature increases, gas density ____________.
decreases
A dramatic shift to the right on a pressure gradient plot for an oil or gas well typically indicates what?
A gas/oil contact, gas/water contact, or oil/water contact
because of the large density variance b/w fluids
fluid pressure gradients being a function of fluid density
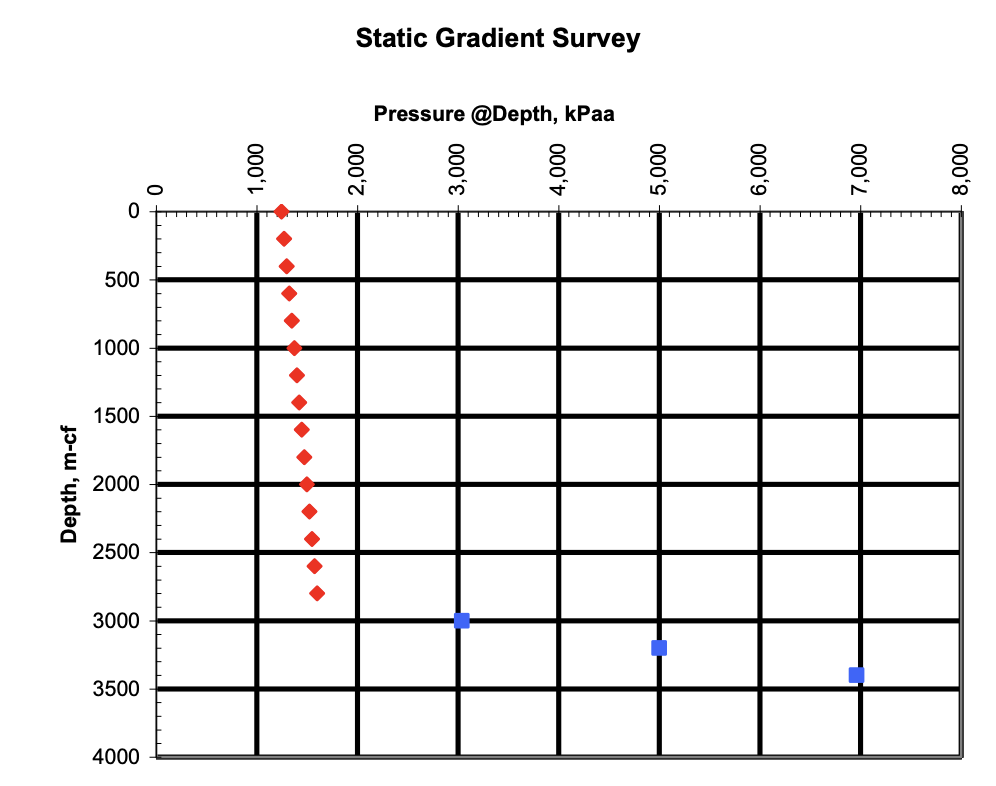
If the slope of a gas pressure gradient is m = 7.9425m/kPaa, what is the gas gradient?
G = 1/m = 1/7.9425 = 0.1259 kPaa/m
obvious from the slope units
Outline the iterative process of calculating Pm (or Pb) used in the Cullender & Smith method

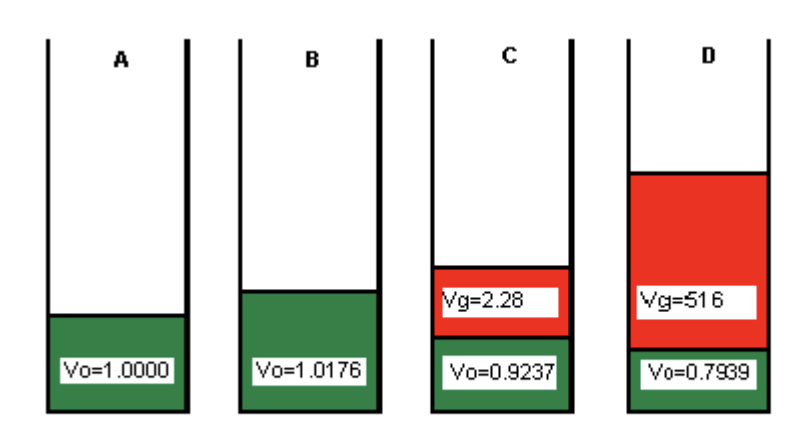
Which cell has the lowest pressure
D
Cells D and E are at the same pressure. Explain the difference in volumes observed.
Cell E must have a lower temperature.
When you cool down oil and gas, both will slightly decrease in volume
The ratio of the volume of oil at reservoir conditions to the volume of oil at standard conditions
Oil Formation Volume Factor, Bo
Metric Unit: rm3/sm3
Imperial Unit: bbl/STB
Bo = (Live Oil Volume)/(Dead Oil Volume)
The ratio of the volume of gas at reservoir conditions to the volume of gas at standard conditions
Gas Formation Volume Factor, Bg
Metric Unit: rm3/sm3
Imperial Unit: ft3/scf
The ratio of the volume of the dissolved gas to the oil volume
Solution gas-oil ratio, Rs
Metric Unit: sm3/sm3
Imperial Unit: SCF/STB
Rs = (Solution Gas Volume @ STP)/(Dead Oil Volume @ STP)
Rs = (Volume of dissolved gas @ STP)/(Volume of oil @ STP)
Can be tricky because the dissolved gas is the volume of gas dissolved in the oil in the reservoir, yet it is stated at standard conditions
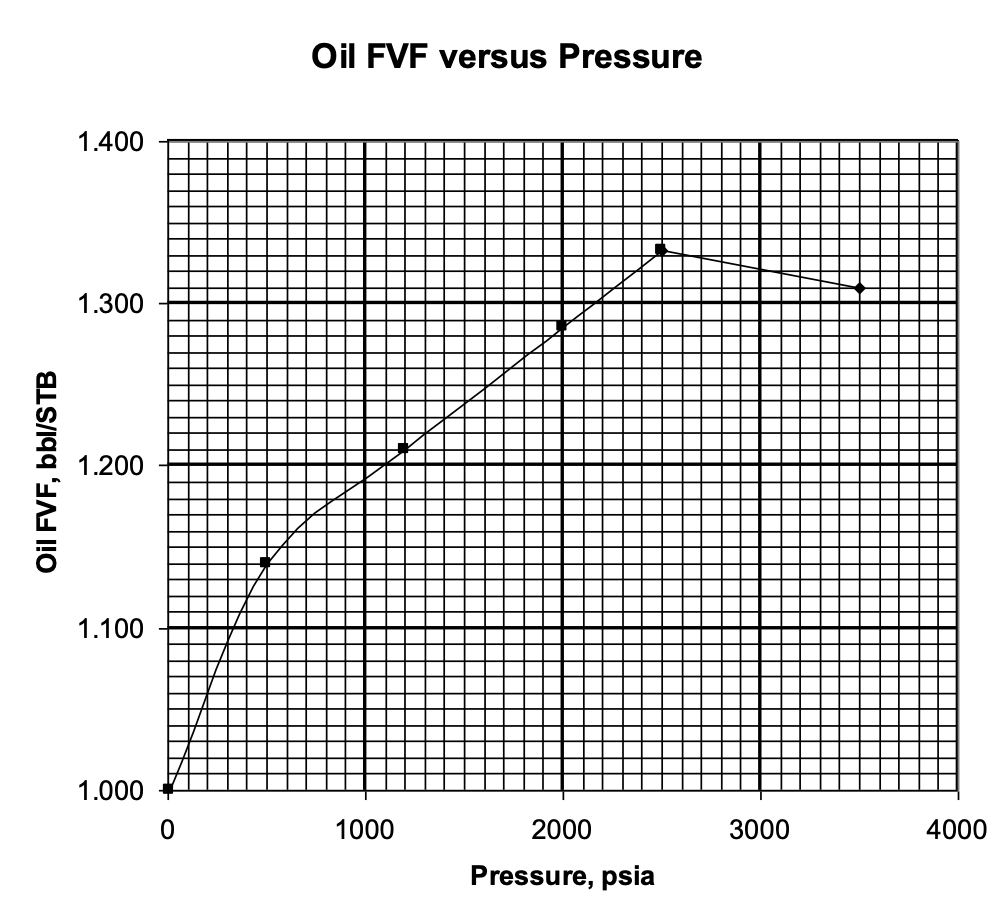
Explain this graph.
Why does Bo rise gradually with increasing pressure?
Why does it drop off slightly at the end?
What is the bubble point pressure?
As reservoir pressure increases, more gas dissolves into the oil, causing the oil to expand, increasing Bo
At the bubble point pressure (the peak on the graph), all of the available free gas has dissolved in the oil (oil is saturated)
Increasing pressure beyond this point will not dissolve additional gas (there is no more free gas)
At pressures above the bubble point, the oil behaves as a slightly compressible liquid. Further increases in pressure cause a small reduction in oil volume, resulting in the slight decrease in Bo observed at the end of the curve

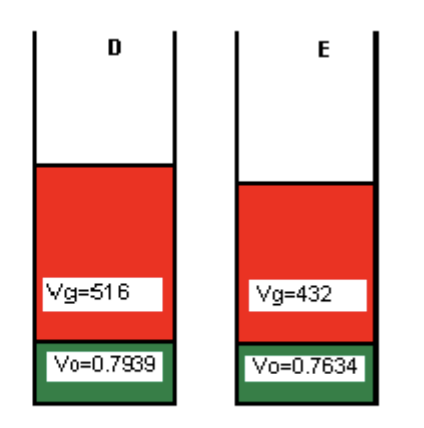
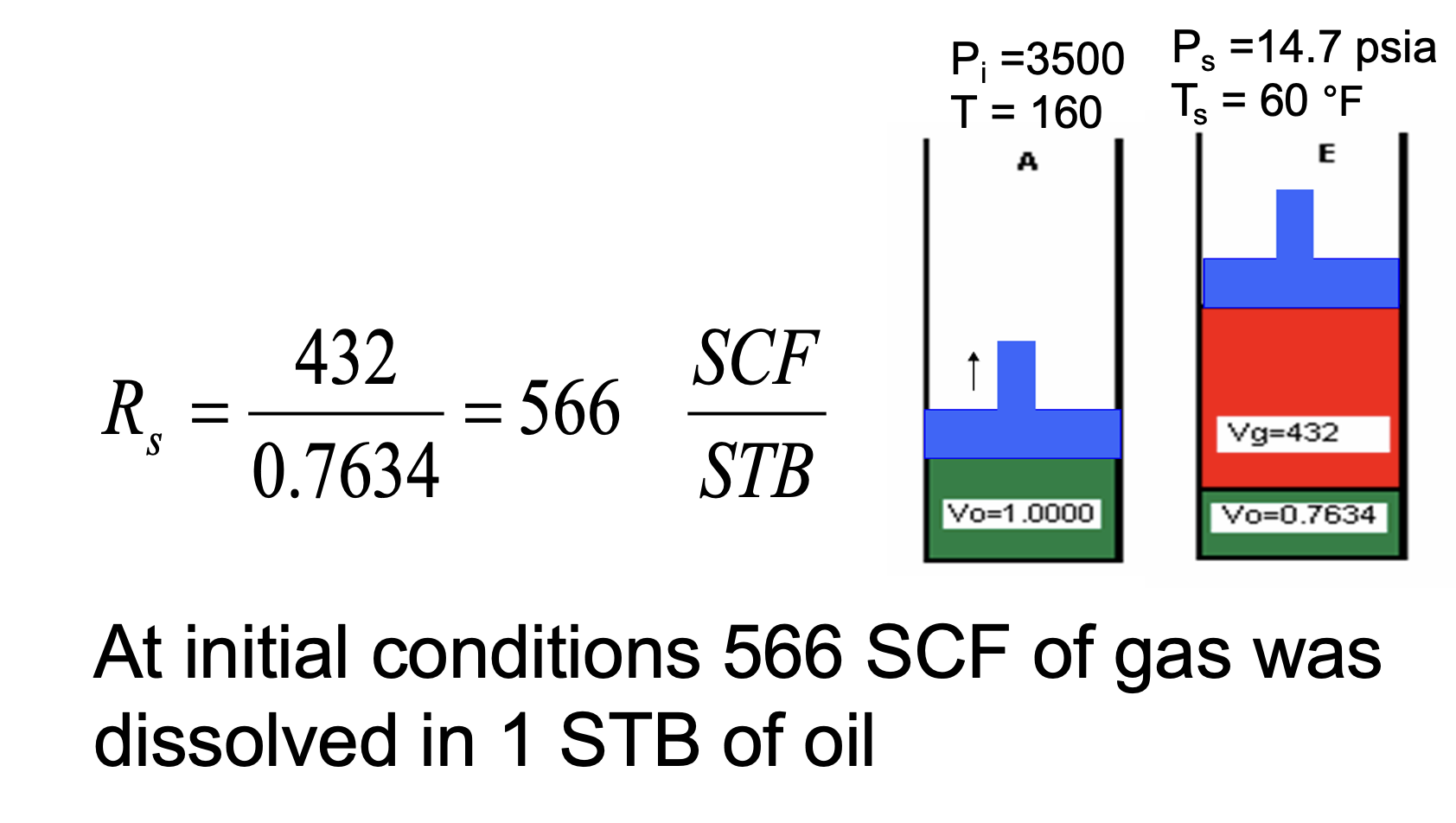
Given that:
Cell E is at standard conditions
In cell C, the Bg = 0.01300 ft3/SCF
What is the solution gas-oil ratio (Rs) for cell C?
Rs = (Solution Gas Volume @ STP)/(Dead Oil Volume @ STP) [formula sheet]
From the image:
Free gas volume for E (STP) = 432 ft3
Dead oil volume @ STP (cell E) = 0.7634 bbl
Free gas volume for C = 2.28 ft3
The remainder of the gas in C is in solution
we need this volume at STP
Convert 2.28 ft3 to STP using Bg
2.28 ft3 / 0.01300 ft3/SCF = 175.38 SCF
Therefore, solution gas volume @ STP in cell C = 432 - 175.38 = 256.62 SCF
So Rs = 256.62 / 0.7634 = 337 SCF/STB
What principle is the material balance equation based on?
Conservation of Mass
TorF: When fluid (oil, gas, water) is produced from the reservoir, that volume of fluid leaves the formation pore space, resulting in a partial void in the original pore space
False
Fluids expand to fill the original pore space
Ve = Vw [formula sheet]
At the bubble point, Pb, what is the volume of free gas in the reservoir?
0
At P > Pb, an oil reservoir is said to be _______________.
undersaturated
all gas is dissolved in solution (no free gas)
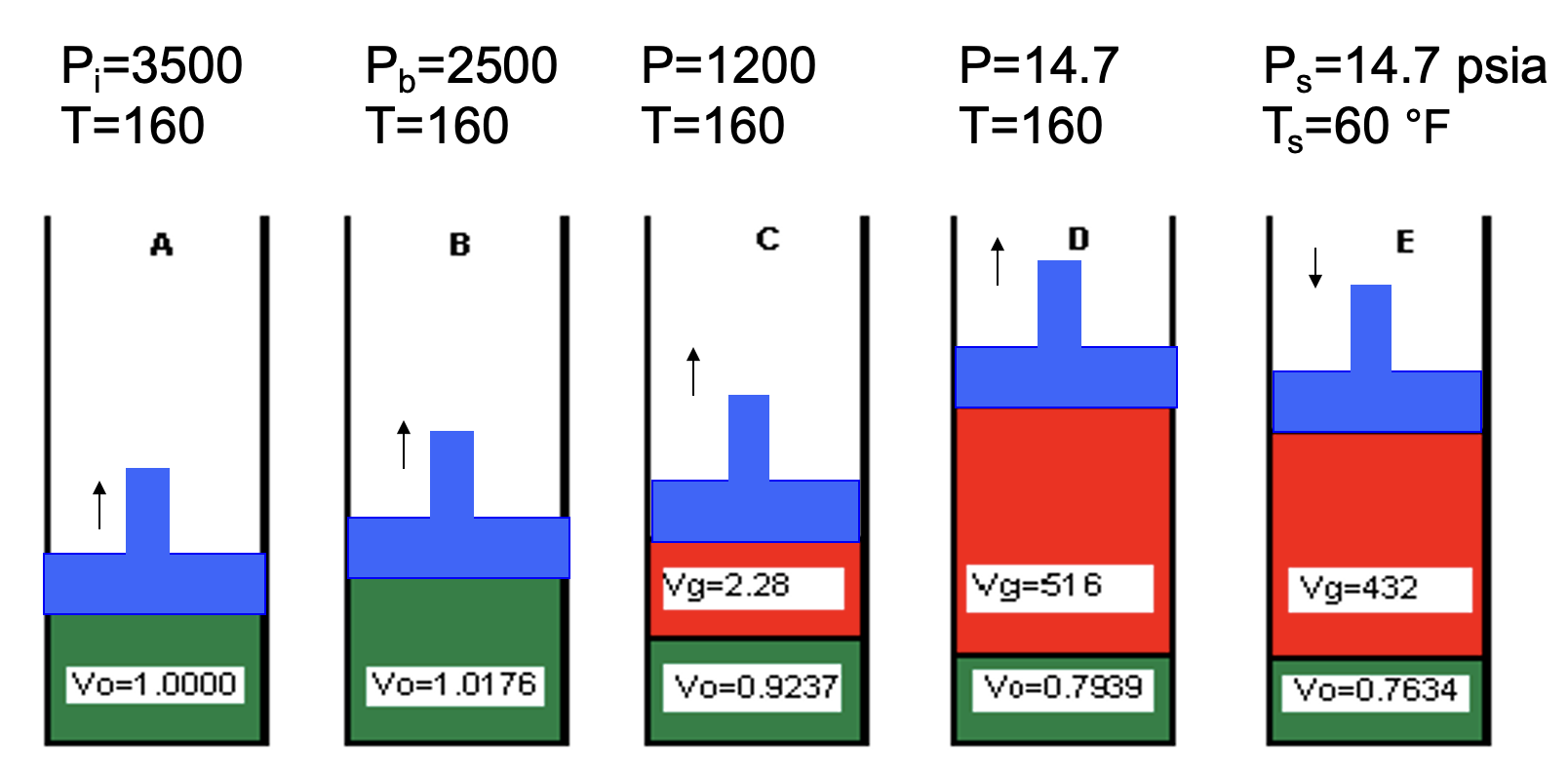
In cell A, what is the volume of gas dissolved in the oil?
What is the Rs @ A?
What is the Rs @ B?
Rs @ B = Rs @ A
because all gas is still in solution
What are the assumptions used in Simple Oil Material Balance?
Initially undersaturated oil
Water expansion & production is ignored
ie. water is incompressible
Define all of these Simple Oil Material Balance parameters:
N
Np
G
Gp
Bo
Bg
Rs
Rp
Ve
Vw
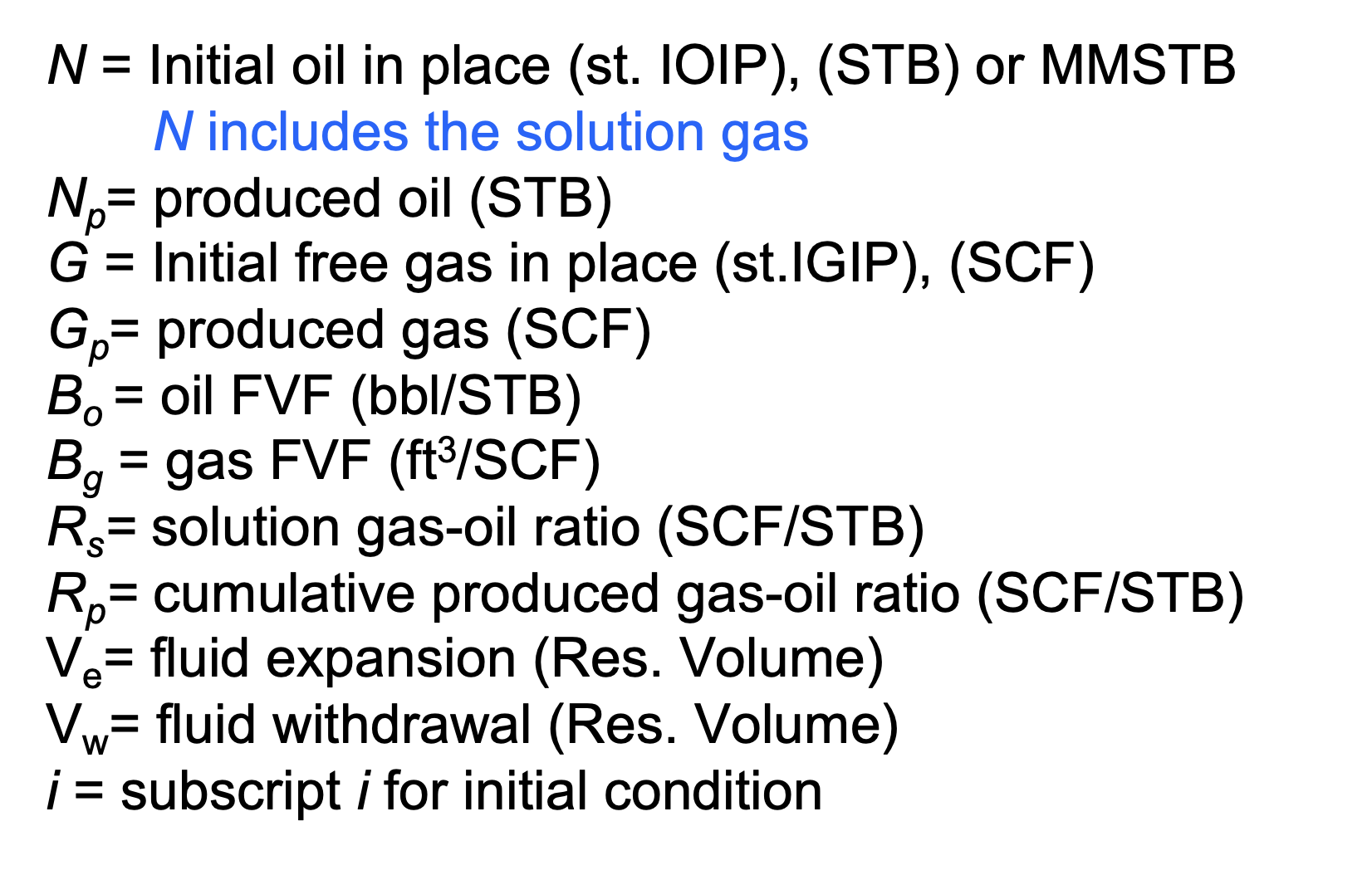
Using Simple Oil MB parameters, and assuming that the reservoir is initially at the bubble point, how would you calculate the total hydrocarbon volume at initial conditions?
N x Boi
At the bubble point all gas is in solution (ie. inside the volume N)
therefore N = total hydrocarbon volume
Recall that N is stated @ standard conditions, so must be adjusted to initial conditions using Boi
Flow in a reservoir that has not yet reached boundary dominated flow is called ________ flow.
transient
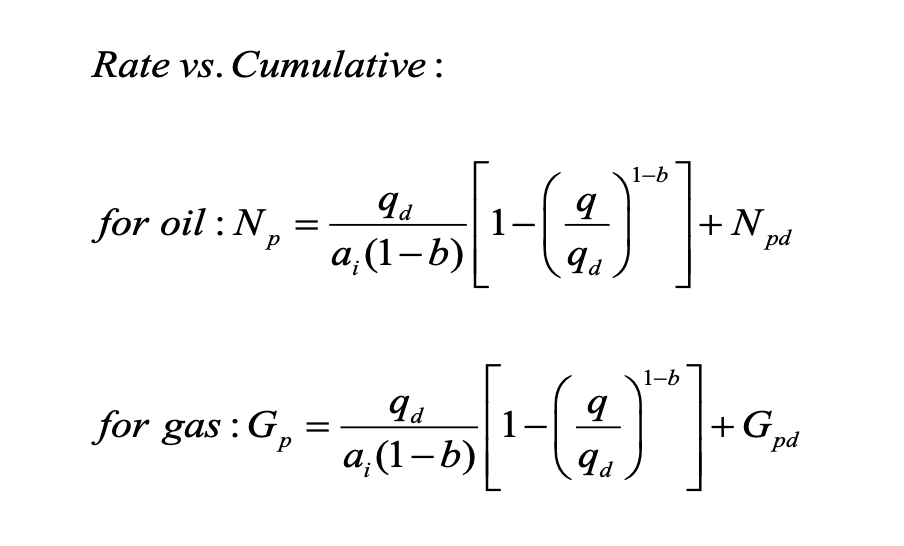
In the Slider Method (hyperbolic) equations shown (for calculating cumulative production), what must the unit of ai be?
1/day
because your rates are all per day
Of the following 4 Oil FVFs, which one represents a heavy oil?
a) Bo = 1.75 (bbl/STB)
b) Bo = 1.25 (bbl/STB)
c) Bo = 1.05 (bbl/STB)
d) Bo = 1.45 (bbl/STB)
c) Bo = 1.05
heaviest oil will always have the lowest Bo (closest to 1)
Heavy oils (dense, low gas solubility) expand very little → smaller Bo
lightest oil will always have the highest Bo
light oils (low density, high gas solubility) expand more due to dissolved gas → larger Bo
You are given the following gas FVFs for different natural gases at reservoir conditions. Which one most likely corresponds to a high pressure, high temperature gas reservoir?
a) Bg = 0.005 (ft3/scf)
b) Bg = 0.02 (ft3/scf)
c) Bg = 0.01 (ft3/scf)
d) Bg = 0.03 (ft3/scf)
a) Bg = 0.005
Bg decreases with increasing pressure and temperature
High P high T gas reservoirs are highly compressed → smaller Bg
Four crude oil samples were analyzed for solution gas–oil ratio at their respective bubble point pressures.
Which sample most likely represents a heavy oil?
a) Rs = 800 (scf/STB)
b) Rs = 50 (scf/STB)
c) Rs = 1500 (scf/STB)
d) Rs = 300 (scf/STB)
b) Rs = 50 (scf/STB)
Heavy oils dissolve very little gas → low Rs
A formation that contains water
Aquifer
The unique combination of temperature and pressure at which the liquid and vapor phases become indistinguishable
Critical Point
How do you calculate the slope on on a semi-log plot?
m = Δln(y)/Δx
A reservoir in which Pi < Pb is referred to as ___________.
Initially saturated
oil with a gas cap (ie. free gas)
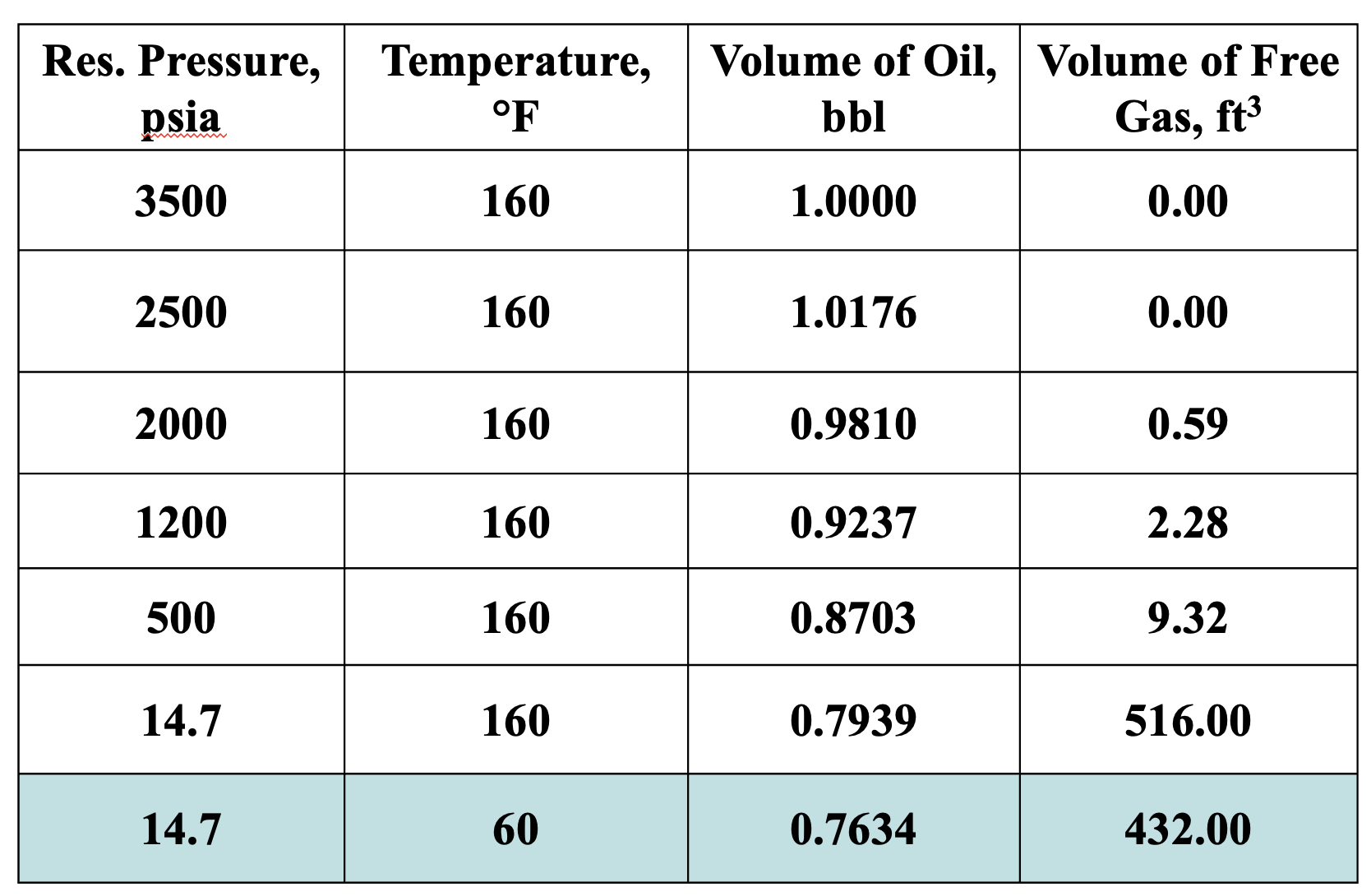
What is the Rso @ standard conditions?
Rso = (total gas - free gas)/(dead oil) = 432/0.7634 = 566 SCF/STB
What is the minimum Rso @ STP?
0
ie. the oil contains no dissolved gas
Does the Rso increase above the bubble point?
No
above the bubble point, all gas is in solution. There is no more available free gas, hence Rso cannot increase