AP Biology Unit 3 - Cellular Energetics
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
50 Terms
Metabolism
The totality of an organism's chemical reactions
Catabolic Pathways
Pathways that release energy by breaking down complex molecules into simpler compounds
Anabolic Pathways
Pathways that consume energy to build complex molecules from similar ones
Thermodynamics
The study of how energy flows through organisms & energy transformations
First Law of Thermodynamics
The energy of the universe is constant; energy cannot be created or destroyed, only transferred and transformed
Second Law of Thermodynamics
Every energy transfer or transformation increases the entropy (disorder) of the universe
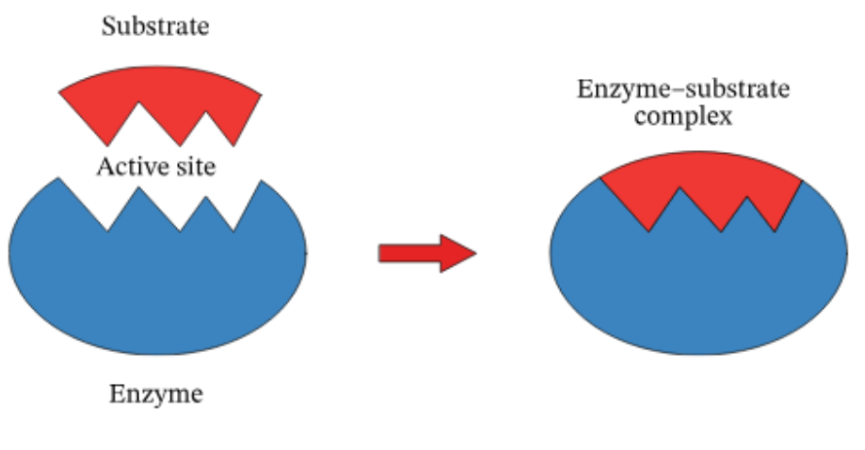
Enzymes
Macromolecules, mostly proteins, & biological catalysts that speed up reactions + are reusable to facilitate synthesis & digestive reactions
must maintain a tertiary shape for functionality
(names often end in “ase” )
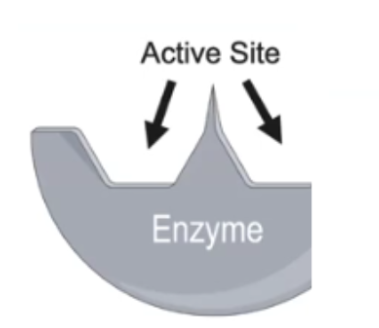
Active Site
An enzyme region, with unique shapes and sizes, that interacts with substrates; their physical and chemical properties must be compatible to interact
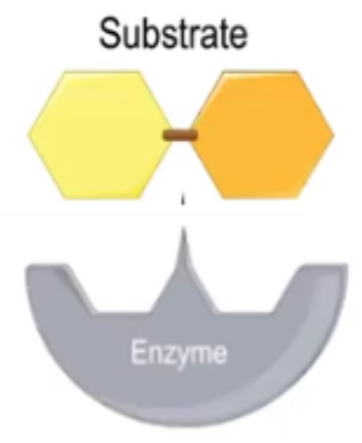
Substrate
A molecule that bonds to an enzyme’s active site; must have compatible physical and chemical properties to interact
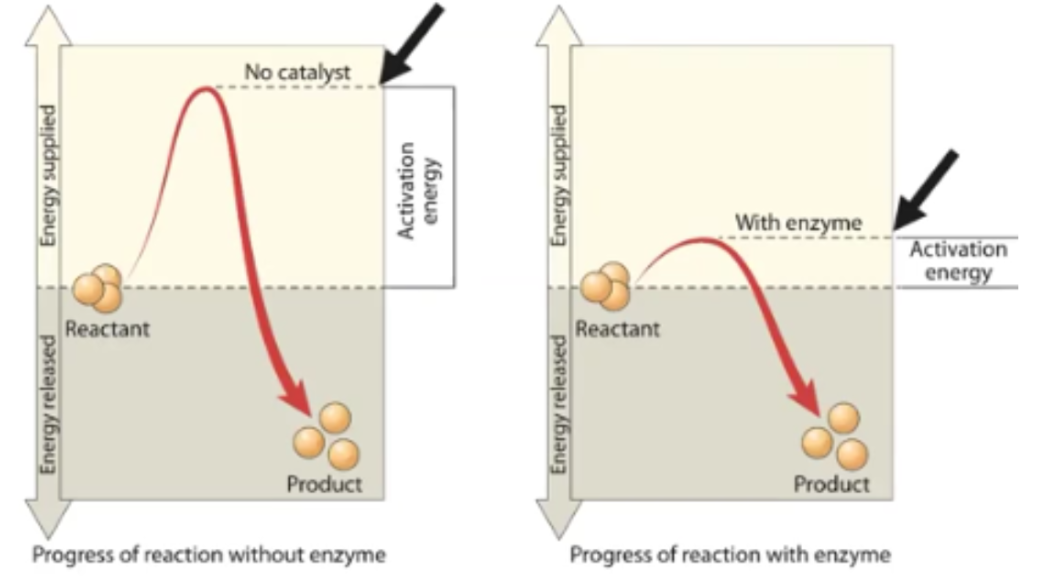
Activation Energy
The initial starting energy that is required by all biochemical reactions; enzymes lower the required activation energy, which accelerates the rate of reaction
Control Test Group
The “baseline data”; a set of data under normal conditions, with no treatment or manipulation
Negative Control Group
A set of data that is not exposed to the experimental treatment
Positive Control Group
A set of data that is exposed to a treatment with a known effect
Experimental Test Group
A set of data under abnormal, treated, or manipulated conditions
Controlled Variables (“Constants”)
Aspects of an experiment that help isolate & identity the impact of a treatment or manipulation
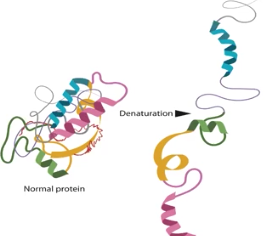
Denaturation
The process of enzymes unraveling due to a change in their conformational shape, which can be caused by a change in their temperature or pH
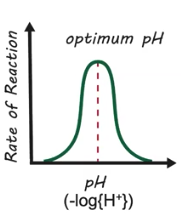
Optimum pH
The pH range where enzyme-mediated reactions occur the quickest
How do changes in pH affect enzyme activity?
Increases & decreases in pH, outside of the optimum pH, both cause denaturation
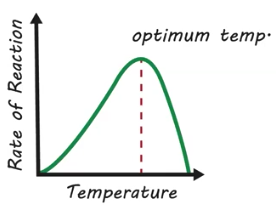
Optimum Temperature
Temperature range where enzyme-mediated reactions occur the quickest
How do changes in temperature affect enzyme activity?
Increases in temperature initially increase the reaction rate but, result in denaturation. Decreases in temperature slow down the reaction and do not cause denaturation.
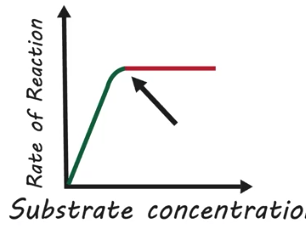
How do changes in substrate concentration affect the rate of reactions?
Increases in substrate concentrations initially increase the reaction rate, since there are more substrate collisions, but a saturation will eventually occur
How do changes in product concentrations affect the rate of reactions?
Increases in product concentration decreases the addition of substrates and lowers the chance of enzyme-substrate collisions
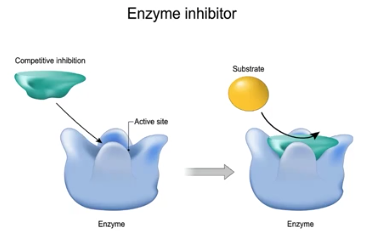
Competitive Inhibitors
Molecules that compete with substrates by binding to enzyme-active sites; slow enzyme activity
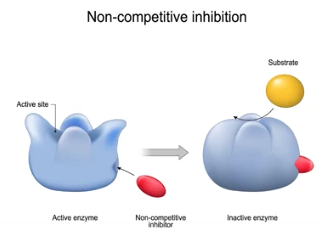
Noncompetitive Inhibitors
Molecules that do not bind to the active site, and instead to the allosteric site, which prevents enzyme function
Allosteric Inhibitor
Molecules that bind to a different enzyme site (any other than the active site) and inhibit its activity, as well as bring a conformational change
Autotrophs
Cells that contain chloroplasts which capture and transform energy from a physical source (sunlight) or chemical source to be useable by all cells
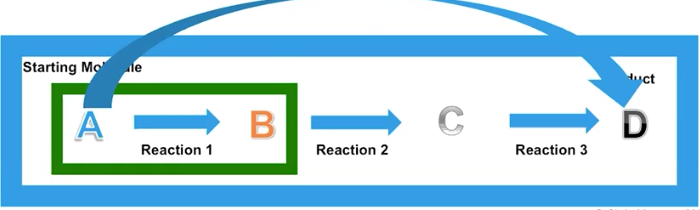
Sequential Pathways/ “Coupling”
The product of the first reaction is the reactant of the next reaction; allow for a more controlled and efficient transfer of energy
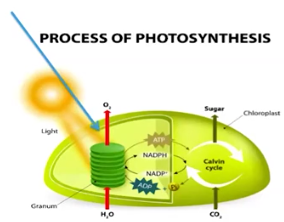
Photosynthesis
The biological process that captures energy from the sun and produces sugars
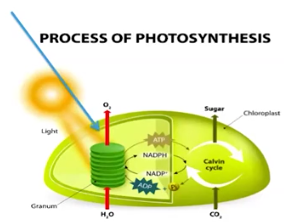
Pathways of Photosynthesis
Photosystems II & I capture energy by using chlorophyll (pigments)
Pigments transform light into chemical energy, which is temporarily stored in NADPH
The electrochemical/proton gradient (caused by H+ ion movement against the gradient in PSI & II) allows ATP synthase to make ATP
ATP & NADPH power the Calvin Cycle
Chlorophylls
Pigments, light-capturing molecules, that capture sunlight and convert it into high-energy electrons; whose energy establishes a proton gradient and reduces NADP+ to NADPH
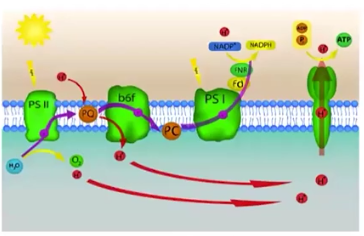
Photosystems
Light-capturing units, embedded in a chloroplast’s thylakoid membrane
Electrochemical/Proton Gradient
A difference in concentration of protons (Hydrogen ions) across a membrane; allows for the presence of ATP Synthase
ATP Synthase
An enzyme that changes ADP to ATP when protons pass through it
How are the products of photosynthesis used in the Calvin Cycle (“the dark reaction”)?
The Calvin Cycle uses ATP, NADPH, & CO2, products of photosynthesis, to produce carbohydrates and organic products, required by plants
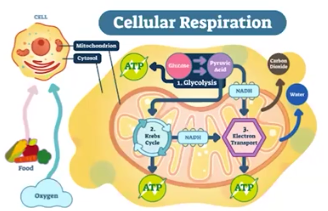
Cellular Respiration
With oxygen; The process of life forms releasing energy from organic materials, such as glucose, to produce and use ATP
Fermentation
Without oxygen; The process of life forms releasing energy from organic materials, such as glucose, to produce and use ATP; creates products of ethanol and lactic acid
Aerobic Respiration
The process of cellular respiration, powered by the presence of oxygen
Anaerobic Respiration
The process of respiration (commonly known as fermentation), not powered by the presence of oxygen
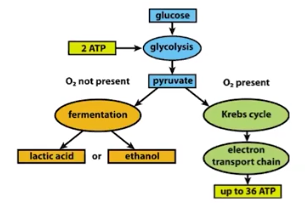
Pathways of Cellular Respiration
Glycolysis= occurs in the cytoplasm
Pyruvate Oxidation= occurs in the mitochondria
Krebs Cycle (the Citric Acid Cycle)= occurs in the mitochondria
Electron Transport Chain= occurs in the mitochondria
Glycolysis
A pathway of fermentation and cellular respiration that occurs in the cytoplasm & releases energy stored in glucose; results in the production of pyruvate, NADH, and a small amount of ATP
Pyruvates
The end-product of glycolysis; when oxidized, enters the Krebs cycle from the mitochondria
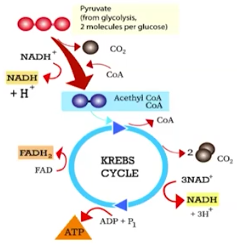
The Krebs Cycle (Citric Acid Cycle)
A pathway of cellular respiration that releases carbon dioxide from organic intermediates, and make a small amount of ATP; occurs in the mitchondria
CO is released from the pyruvates
High energy electrons are transferred to NADH+ and FADH2 carriers
ADP is phosphorylated forming ATP
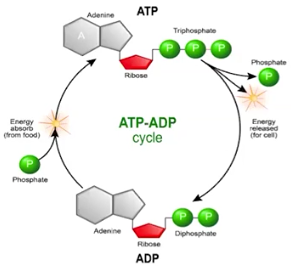
ATP-ADP Cycle
Through the conversion of ATP to ADP, energy is released from ATP when chemical bonds are broken between the 2nd and 3rd phosphate
Electron Transport Chain (ETC)
Occurring in the mitochondria/chloroplast membrane, the final pathway of cellular respiration, whose function is to transfer energy from electrons; membrane proteins make up its chain and facilitate the conserved reaction
Role of NADH and FADH2 in the ETC
NADH and FADH2 are electron carries that carry electrons to the ETC and facilitate the active transport of protons (hydrogen ions), which establishes an electrochemical/proton gradient
Chemiosmosis
The diffusion and active transport of hydrogen ions/protons across a selectively permeable membrane; the flow of protons through the ATP Synthase powers the synthesis of ATP
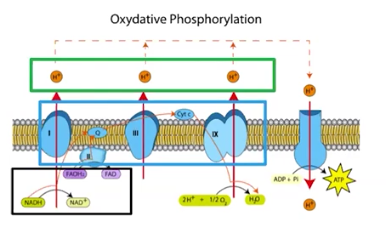
Oxidative Phosphorylation
The process of synthesizing ATP, using the stored energy of a proton gradient
Oxidation is the process of NADH and FADH2 giving high-energy electrons to the ETC
Phosphorylation is the process of electron entering ATP Synthase
Importance of Decoupling
Decoupling refers to the proton gradient not being used by ATP synthase to produce ATP, which causes the energy stored in the electrochemical/proton gradient to be released & generate heat. This can be used to regulate body temperature.
Fitness
Term for an individual organism’s ability to survive and reproduce
How does molecular variation increase the ability of cells?
The more variation in a cell’s number of & type of molecules & structure, the more fitness it has. Variation gives organisms a better chance to survive in changing conditions
(ex. Chloroplasts can capture different wavelengths of light → Photosynthesis is flexible during different times of the day & year)