Honors Chemistry Unit 1 Test
5.0(2)
Card Sorting
1/104
Earn XP
Description and Tags
Last updated 3:05 AM on 9/12/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
105 Terms
1
New cards
Change a speed of 72.4 miles per hour to its equivalent in meters per second.
32\.7 m/s
2
New cards
Convert 45.3 cm to its equivalent measurement in mm.
453 mm
3
New cards
A block occupies 0.2587 ft^3. What is its volume in mm^3?
7\.326 x 10^6 mm^3
4
New cards
A block of silver-colored metal with a volume of 65.0 cm^3 has a mass of 750.0 g. The density of pure silver is 10.5 g/cm^3. Is the metal pure silver?
11\.5 g/cm^3
The metal is not pure silver
The metal is not pure silver
5
New cards
4\.15 cm × 1.8 cm = ?
7\.5cm^2
6
New cards
13\.00 m − 0.54 m = ?
12\.46 m
7
New cards
(1.7 × 10^-5 m) ⁄ (3.72 × 10^-4 m) = ?
6.3 x 10^-9 m^2
8
New cards
The following length measurements were taken by students using several different measuring devices. Find the average of the measurements. Make sure that your answer has the correct number of significant figures. \n 10.05 cm, 10.1 cm, 9.741 cm, 10.6 cm, 10.5 cm
10\.2 cm
9
New cards
Round off the measurement 0.0030955 m to three significant figures.
0\.00310 m
10
New cards
What is the sum of 2.7 g and 2.47 g expressed in the correct number of significant digits?
5\.2 g
11
New cards
What is the product of the number 1000 and the measurement 0.00357 m expressed in the correct number of significant digits?
4 m
12
New cards
Perform the following operation: 3.43 cm x 5.2 cm. Make sure that your answer has the correct number of significant figures.
18 cm^2
13
New cards
Express 0.05 grams in kilograms, using the correct abbreviations
5 x 10^-5
14
New cards
What is the density of an object having a mass of 4.0 g and a volume of 39.0 cubic centimeters?
0\.10 g/cm^3
15
New cards
What is the volume of an object with a density of 7.73 g/cm^3 and a mass of 5.40 x 10^2 g?
69\.9 cm^3
16
New cards
The density of osmium, which is the densest metal, is 22.57 g/cm^3. What is the mass of a block of osmium that measures 1.00 cm x 4.00 cm x 2.50 cm?
226 g
17
New cards
68\.9 (2)
1. Rounded
2. Rounded Result Expressed in Scientific Notation
1. Rounded
2. Rounded Result Expressed in Scientific Notation
1. 69
2. 6.9 x 10^1
18
New cards
0\.00923 (2)
1. Rounded
2. Rounded Result Expressed in Scientific Notation
1. Rounded
2. Rounded Result Expressed in Scientific Notation
1. 0.0092
2. 9.2 x 10^-3
19
New cards
624 (2)
1. Rounded
2. Rounded Result Expressed in Scientific Notation
1. Rounded
2. Rounded Result Expressed in Scientific Notation
1. 620
2. 6.3 x 10^2
20
New cards
the study of the processes that take place in organisms
biochemistry
21
New cards
the study of all chemicals containing carbon
organic chemistry
22
New cards
the study of chemicals that, in general, do not contain carbon
inorganic chemistry
23
New cards
concerned with the mechanism, rate, and energy transfer that occurs when matter undergoes a change
physical chemistry
24
New cards
the study of the composition of matter
analytical chemistry
25
New cards
composed of two or more substances chemically combined in a fixed proportion
compound
26
New cards
process in which substances are changed into different substances
chemical reaction
27
New cards
substance that cannot be changed into simpler substances by chemical means
element
28
New cards
a process in which a liquid is boiled to produce a vapor that is condensed again into a liquid
distillation
29
New cards
describes mixture with a uniform composition
homogeneous
30
New cards
amount of matter an object contains
mass
31
New cards
known or estimated in a measurement
significant figure
32
New cards
the non-SI scale for temperature
Celcius
33
New cards
closeness to true value
accuracy
34
New cards
narrowness of range of measurements
precision
35
New cards
the SI scale for temperature
Kelvin
36
New cards
the force of gravity on an object
weight
37
New cards
the lowest point on the Kelvin scale
absolute zero
38
New cards
a substance formed in a chemical reaction
product
39
New cards
gaseous state of substance that is a liquid or solid at room temperature
vapor
40
New cards
a physical blend of two or more components
mixture
41
New cards
not uniform in composition
heterogeneous mixture
42
New cards
starting substance in a chemical reaction
reactant
43
New cards
part of a sample having uniform composition and properties
phase
44
New cards
Which field of science studies the composition and structure of matter?
chemistry
45
New cards
The study of chemicals that, in general, do not contain carbon is traditionally called what type of chemistry?
inorganic
46
New cards
A theory is a ____.
well-tested explanation for a broad set of observations
47
New cards
A hypothesis is a ____.
proposed explanation for an observation
48
New cards
What is a good example of chemical technology?
development of a coating for non-stick frying pans
49
New cards
The variable that is observed during an experiment is called what type of variable?
dependent
50
New cards
What are examples of things that are considered physical properties of a substance?
color, odor, melting/boiling points, malleability, and hardness
51
New cards
Which state of matter takes both the shape and volume of its container?
gas
52
New cards
Which state of matter is characterized by having a definite shape and a definite volume?
solid
53
New cards
All of the following are physical properties of a substance in the liquid state EXCEPT ____.
a. indefinite volume
b. definite mass
c. not easily compressed
d. indefinite shape
a. indefinite volume
b. definite mass
c. not easily compressed
d. indefinite shape
a. indefinite volume
54
New cards
Which of the following is a heterogeneous mixture?
a. air
b. salt water
c. steel
d. soil
a. air
b. salt water
c. steel
d. soil
d. soil
55
New cards
Which of the following CANNOT be considered a single phase?
a. a pure solid
b. a pure liquid
c. a homogeneous mixture
d. a heterogeneous mixture
a. a pure solid
b. a pure liquid
c. a homogeneous mixture
d. a heterogeneous mixture
d. a heterogeneous mixture
56
New cards
A substance that can be separated into two or more substances only by a chemical change is a(n) ____.
compound
57
New cards
Which of the following represents a compound?
Question 34Select one:
a. H
b. *H-3*
c. H2O
d. O-16
Question 34Select one:
a. H
b. *H-3*
c. H2O
d. O-16
c. H2O
58
New cards
What must occur for a change to be a chemical reaction?
There must be a change in chemical properties.
59
New cards
Which of the following does NOT involve a physical change?
a. mixing
b. melting
c. grinding
d. decomposing
a. mixing
b. melting
c. grinding
d. decomposing
d. decomposing
60
New cards
Which of the following does NOT indicate that a chemical change may have taken place?
a. fracture formation
b. gas production
c. precipitate formation
d. energy transfer
a. fracture formation
b. gas production
c. precipitate formation
d. energy transfer
a. fracture formation
61
New cards
What happens to matter during a chemical reaction?
Matter is neither destroyed or created.
62
New cards
What is true for all chemical reactions?
The total mass of the reactants equals the total mass of the products.
63
New cards
Which of the following is a formula?
a. Fe
b. Water
c. H2O
d. Fluorine
a. Fe
b. Water
c. H2O
d. Fluorine
c. H2O
64
New cards
In which state of matter do the particles tend to be most closely packed?
solid
65
New cards
Which of the following could be separated by **physical** means?
a. salt water
b. silver
c. sodium chloride
d. rust
a. salt water
b. silver
c. sodium chloride
d. rust
a. salt water
66
New cards
Matter is anything that has
mass and occupies space.
67
New cards
The separation of two liquids made possible by a difference in the boiling points of each is
distillation
68
New cards
What would be considered to be unsafe in lab?
horseplay
69
New cards
Which of the following would be best to use for heating water to a boil?
a. watch glass
b. rubber policeman
c. beaker
d. Satchmo
a. watch glass
b. rubber policeman
c. beaker
d. Satchmo
c. beaker
70
New cards
The expression of 5008 km in scientific notation is ____.
5\.008 × 10^3 km
71
New cards
What is the result of multiplying 2.5 x 10^10 by 3.5 x 10^-7?
8\.75 × 10^3
72
New cards
Express the sum of 1111 km and 222 km using the correct number of significant digits.
1333 km
73
New cards
Express the sum of 7.68 m and 5.0 m using the correct number of significant digits
12\.7 m
74
New cards
Express the product of 4.0 x 10^-2 m and 8.1 x 10^2 m using the correct number of significant digits.
3\.2 x 10^1
75
New cards
When multiplying and dividing measured quantities, the number of significant figures in the result should be equal to the number of significant figures in ____.
the least precise measurement
76
New cards
What is the volume of a salt crystal measuring 2.44 x 10^-2 m by 1.4 x 10^-3 m by 8.4 x 10^-3 m?
2\.9 × 10^–7 m^3
77
New cards
What is the temperature –34C expressed in kelvins?
239 K
78
New cards
A cubic meter is about the same as the volume occupied by a ____.
washing machine
79
New cards
The quantity 44 liters expressed in cubic meters is ____.
0\.044 m^3
80
New cards
Density is found by dividing ____.
mass by volume
81
New cards
What is the density of an object having a mass of 8.0 g and a volume of 25 cm^3?
\n 0.32 g/cm^3
82
New cards
What is the volume of 80.0 g of ether if the density of ether is 0.70 g/mL?
1\.1 x 10^2 mL
83
New cards
What is the volume of 45.6 g of silver if the density of silver is 10.5 g/mL?
4\.34 mL
84
New cards
If a liter of water is heated from 20C to 50C, what happens to its volume?
The volume increases.
85
New cards
If the temperature of a piece of steel decreases, what happens to its density?
The density increases.
86
New cards
As the density of a substance increases, the volume of a given mass of that substance ____.
decreases
87
New cards
Discuss the relationship between the terms hypothesis, scientific theory, and scientific law as each relates to observations.
A hypothesis is a proposed explanation for an observation. A scientific theory is a well-tested explanation of a broad set of observations. A scientific law is a rule that is always true which explains an observation
88
New cards
What is the difference between a solution and a heterogeneous mixture? Give 2 examples of each and explain why each example falls into the category you listed.
A solution is a homogeneous mixture of two or more substances where the particles of one substance are evenly dispersed throughout the other substance(s). The components of a solution cannot be easily separated by physical means.
Ex: Saltwater, Air
A heterogeneous mixture is a combination of two or more substances that are not evenly mixed and can be easily separated by physical means.
Ex: Oil and Water, Soil
Ex: Saltwater, Air
A heterogeneous mixture is a combination of two or more substances that are not evenly mixed and can be easily separated by physical means.
Ex: Oil and Water, Soil
89
New cards
Give an example of a two-phase mixture and describe how you would separate the substances.
An example of a two-phase mixture is sand and water. To separate the sand and water, I would bring the mixture to a boil, so the water would evaporate out, and the sand would stay in the container.
90
New cards
Explain the difference between an element and a compound. Include examples in your explanation.
An element is the simplest form of matter that has a unique set of properties. A compound is a substance that contains two or more elements chemically combined in a fixed proportion. An element cannot be broken down into simpler components through chemical reactions. Compounds can be broken down into simpler substances through chemical reactions.
Ex: H2O (water) is a compound, and it can be broken down into two elements (H2) and (O) through a chemical reaction.
Ex: H2O (water) is a compound, and it can be broken down into two elements (H2) and (O) through a chemical reaction.
91
New cards
What does the chemical formula of a compound indicate? Use examples to illustrate your explanation.
The chemical formula of a compound indicates the type of element and how much of it is in the compound. For example, the chemical formula for water (H2O) reveals that there are 2 Hydrogen atom and 1 Oxygen atom in a water molecule.
92
New cards
Explain the difference between chemical properties and physical properties. Give an example of each.
Physical properties can be measured without needing to make a chemical change to the substance.
Ex: Color, Melting/Boiling Point, Density, and Hardness
Chemical properties can only be measured and observed by making chemical changes to the substance on a molecular scale.
Ex: Heat of Combustion, Rusting, Burning
Ex: Color, Melting/Boiling Point, Density, and Hardness
Chemical properties can only be measured and observed by making chemical changes to the substance on a molecular scale.
Ex: Heat of Combustion, Rusting, Burning
93
New cards
Discuss the difference between physical changes and chemical changes. Use an example to illustrate the difference.
A physical change is any change that does not alter the identity of the substance. A chemical change is any change that changes the identity of the substance. (Can also be called chemical reactions)
Ex: A physical change is boiling, but a chemical change is combustion. Combustion is irreversible, and boiling is reversible.
Ex: A physical change is boiling, but a chemical change is combustion. Combustion is irreversible, and boiling is reversible.
94
New cards
State the Law of Conservation of Mass.
If the Law of Conservation of Mass appears to be violated, what is likely to be the explanation?
What would the total mass of products be if 10 grams of water decomposes to produce hydrogen and oxygen?
If the Law of Conservation of Mass appears to be violated, what is likely to be the explanation?
What would the total mass of products be if 10 grams of water decomposes to produce hydrogen and oxygen?
The Law of Conservation of Mass states that mass cannot be created nor destroyed.
The likely explanation of a violation of the law of conservation of mass is that the substance changed states of matter into a gas and escaped into the atmosphere.
The total mass of the products would be 10 grams
The likely explanation of a violation of the law of conservation of mass is that the substance changed states of matter into a gas and escaped into the atmosphere.
The total mass of the products would be 10 grams
95
New cards
Explain the difference between the Celsius and Kelvin temperature scales. Use comparable temperatures in your discussion.
Celcius is used by people around the world, and Kelvin is used by scientists. The difference between the C and Kelvin is 273 degrees. Ex: Water freezes at 0 degrees C and at 273 degrees Kelvin.
96
New cards
Explain the difference between mass and weight. Provide an example to illustrate the distinction.
Mass is the amount of matter an object contains and weight is a force that measures the pull of gravity on a given mass.
Ex: On the moon, your mass is the same, but your weight is different due to the weaker gravity pull.
Ex: On the moon, your mass is the same, but your weight is different due to the weaker gravity pull.
97
New cards
Why is the metric system the preferred system of measurement for science?
Because it is a base 10 system, and it is universally used.
98
New cards
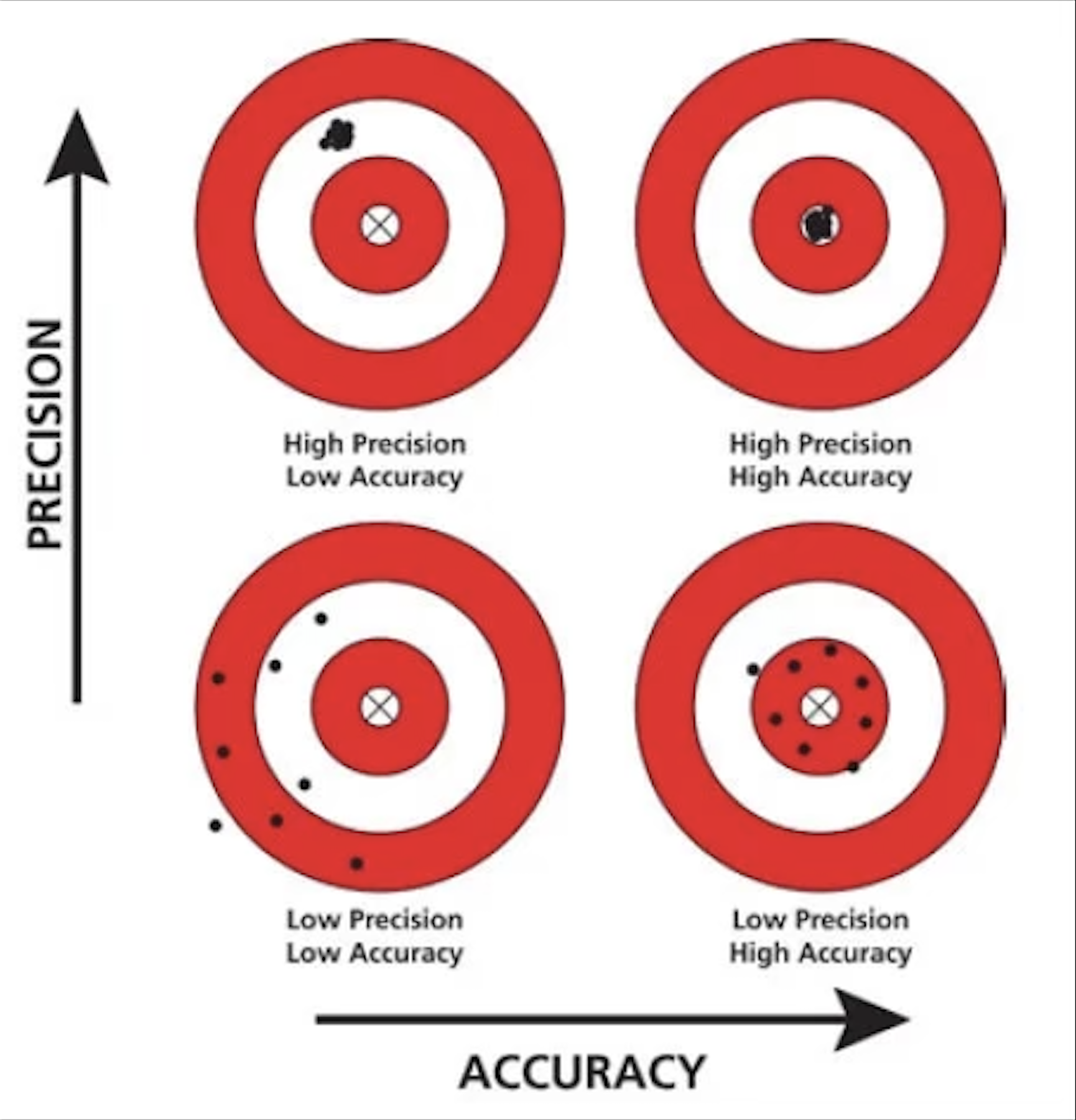
Explain the difference between precision and accuracy. Suppose you made three different mass measurements of a sugar sample you knew to have a mass of 1 g. How would you know whether or not the measurements were accurate? How would you know whether or not they were precise? Could the three measurements be precise, but not accurate? Explain.
Accuracy is the degree of closeness to the true value. Precision is the degree to which an instrument or process will repeat the same value.
Ex: If the 3 measurements were all around the same number then it is precise, but if they are all close to 1 g then the measurements are accurate. Yes, the measurements can be precise but not accurate: if the numbers were all close to 2 g, then it would be a precise measurement, but because they are not close to 1 g, the measurement is not accurate.
Ex: If the 3 measurements were all around the same number then it is precise, but if they are all close to 1 g then the measurements are accurate. Yes, the measurements can be precise but not accurate: if the numbers were all close to 2 g, then it would be a precise measurement, but because they are not close to 1 g, the measurement is not accurate.
99
New cards
What is an extensive property?
a property that depends on the amount of matter in a sample
100
New cards
What is an intensive property?
a property that DOES NOT depend on the amount of matter in a sample