CHEM 1101: Skills Check #2
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms

The most precise glassware on the list to measure a small volume.
Glass Waste
Solid Chemical Waste
Liquid Chemical Waste
Trash Can
Sink
50 mL Beaker
50 mL Graduated Cylinder
10 mL Graduated Pipet
10 mL Graduated Cylinder
50 mL Volumetric Flask
Answer: 10 mL Graduated Pipet
Explanation:
The following are disposal locations, not measuring tools.
Glass Waste
Solid Chemical Waste
Liquid Chemical Waste
Trash Can
Sink
50 mL Beaker
Not precise, volume markings are a rough estimate.
50 mL Graduated Cylinder
Measures volume, but it’s meant for larger amounts. The scale spacing makes small volumes less precise.
10 mL Graduated Pipet
Designed specifically to measure and transfer small volumes accurately, with fine graduations.
10 mL Graduated Cylinder
Better than a 50 mL cylinder for small volumes, but still not as precise as a pipet.
50 mL Volumetric Flask
Very precise for making one solution to exactly 50.0 mL, not for measuring different small volumes.

Where you dispose of extra unreacted Zn from the copper cycle experiment.
Glass Waste
Solid Chemical Waste
Liquid Chemical Waste
Trash Can
Sink
50 mL Beaker
50 mL Graduated Cylinder
10 mL Graduated Pipet
10 mL Graduated Cylinder
50 mL Volumetric Flask
Answer: Solid Chemical Waste
Explanation:
Unreacted Zn = Leftover solid zinc metal from the copper cycle
Glass Waste
It’s not glass.
Solid Chemical Waste
The unreacted zinc is a solid chemical.
Liquid Chemical Waste
It’s a solid metal, not a liquid.
Trash Can
Lab metals and chemicals never go in the trash or down the sink unless your manual says so. The copper cycle manual also says waste goes in proper containers, never the drain.
Sink
“Only water down the drain. All other chemicals and materials should be disposed of properly. When in doubt, ask.”
Where you dispose of extra water that you didn't need for your parallel dilutions.
Glass Waste
Solid Chemical Waste
Liquid Chemical Waste
Trash Can
Sink
50 mL Beaker
50 mL Graduated Cylinder
10 mL Graduated Pipet
10 mL Graduated Cylinder
50 mL Volumetric Flask
Answer: Sink
Explanation:
This refers to the clean water you measured out to do your parallel dilutions. It hasn’t been mixed with dye or any other chemical yet.
Lab glassware options aren’t disposal locations.
Glass Waste
This is for broken glass.
Solid Chemical Waste
This is for solid chemicals or contaminated solids.
Liquid Chemical Waste
Since it’s just unused water, it’s not contaminated and not hazardous.
Trash Can
This is for regular dry trash, not liquids.
Sink
Clean, unused water is safe to pour down the drain.
Where you dispose of your aspirin sample that remains after you have used part of it to analyze.
Glass Waste
Solid Chemical Waste
Liquid Chemical Waste
Trash Can
Sink
50 mL Beaker
50 mL Graduated Cylinder
10 mL Graduated Pipet
10 mL Graduated Cylinder
50 mL Volumetric Flask
Answer: Solid Chemical Waste
Explanation:
After you analyze part of your aspirin, the rest is still a chemical sample. It’s a solid organic compound, even if it seems harmless
Glass Waste
This is only for broken or disposable glass, not chemical solids.
Solid Chemical Waste
This is for solid chemicals or contaminated solids.
Liquid Chemical Waste
The remaining aspirin sample isn’t a liquid.
Trash Can
This is for regular dry trash, not liquids.
Sink
Lab-made chemicals never go in the trash can.
Where you dispose of extra hydrochloric acid left over after an experiment.
Glass Waste
Solid Chemical Waste
Liquid Chemical Waste
Trash Can
Sink
50 mL Beaker
50 mL Graduated Cylinder
10 mL Graduated Pipet
10 mL Graduated Cylinder
50 mL Volumetric Flask
Answer: Liquid Chemical Waste
Explanation:
Glass Waste
This is only for broken or disposable glass.
Solid Chemical Waste
This is for solid chemicals or contaminated solids.
Liquid Chemical Waste
Extra hydrochloric acid is a liquid acid reagent left after the experiment.
Trash Can
This is for regular non-chemical trash.
Sink
“Only water down the drain. All other chemicals and materials should be disposed of properly. When in doubt, ask.”
Where you dispose of chemicals that you scrape off of your filter paper.
Glass Waste
Solid Chemical Waste
Liquid Chemical Waste
Trash Can
Sink
50 mL Beaker
50 mL Graduated Cylinder
10 mL Graduated Pipet
10 mL Graduated Cylinder
50 mL Volumetric Flask
Answer: Solid Chemical Waste
Explanation:
Glass Waste
This is only for broken or disposable glass.
Solid Chemical Waste
Chemicals scraped off filter paper are solid residues.
Liquid Chemical Waste
Extra hydrochloric acid is a liquid acid reagent left after the experiment.
Trash Can
This is for regular non-chemical trash.
Sink
“Only water down the drain. All other chemicals and materials should be disposed of properly. When in doubt, ask.”
Where you dispose of a test tube that broke in the centrifuge.
Glass Waste
Solid Chemical Waste
Liquid Chemical Waste
Trash Can
Sink
50 mL Beaker
50 mL Graduated Cylinder
10 mL Graduated Pipet
10 mL Graduated Cylinder
50 mL Volumetric Flask
Answer: Glass Waste
Explanation:
The main issue is broken glass.
In lab safety, any broken glassware goes in the glass waste or broken glass container. This is to protect custodial staff and prevent puncture injuries.
The prompt doesn’t say the test tube had hazardous chemicals in it; it just says it broke in the centrifuge. So we follow the standard broken glass rule.
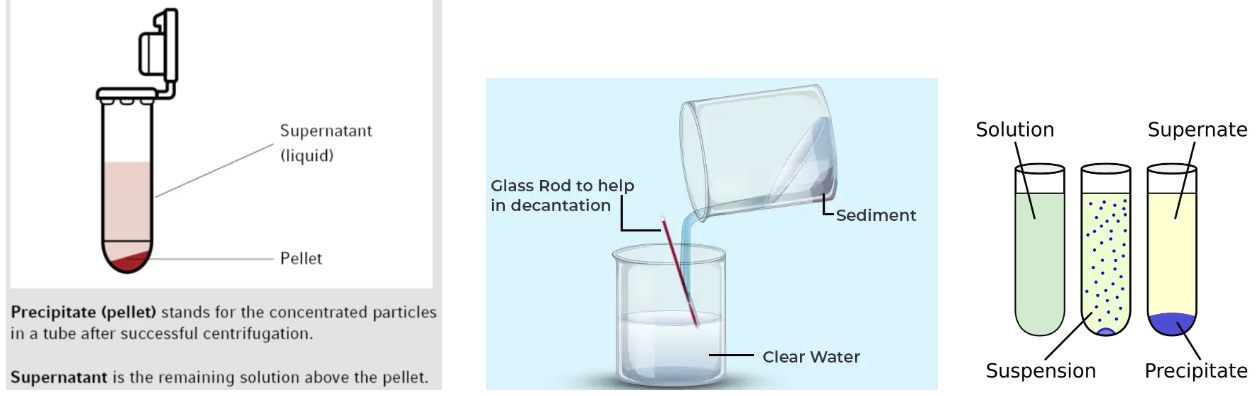
Where you dispose of the supernantent that you decanted in the copper cycle lab
Glass Waste
Solid Chemical Waste
Liquid Chemical Waste
Trash Can
Sink
50 mL Beaker
50 mL Graduated Cylinder
10 mL Graduated Pipet
10 mL Graduated Cylinder
50 mL Volumetric Flask
Answer: Liquid Chemical Waste
Explanation:
In the copper cycle, the supernatant you decant after heating with sodium hydroxide (NaOH) is the leftover liquid sitting above the solid.
Lab Manual: NOTE: The supernatant is mostly basic, so we are keeping it separate from the rest of the waste. Please place the waste from this step in the container with a label that includes “sodium hydroxide 10%”
That labeled container is a liquid waste container, just a specific one for basic sodium hydroxide (NaOH) containing liquid.

You should pour from a stock bottle in the hood into this glassware.
Glass Waste
Solid Chemical Waste
Liquid Chemical Waste
Trash Can
Sink
50 mL Beaker
50 mL Graduated Cylinder
10 mL Graduated Pipet
10 mL Graduated Cylinder
50 mL Volumetric Flask
Answer: 50 mL Beaker
Explanation:
Stock Bottle = The big shared reagent bottle that stays in the fume hood. You don’t take it to your bench, and you try to minimize contamination.
When you pour out of a stock bottle, you want something:
Wide-mouthed, so you don’t miss and spill.
Easy to rinse if a little extra goes in.
Not used for precise measuring yet.
That points to a simple beaker.
Graduated cylinders and pipets are for measuring after you have a smaller working amount. You shouldn’t pour directly into them from a stock bottle because it’s easy to overshoot and contaminate the stock bottle’s rim.
Volumetric flasks are for making solutions to an exact volume. You usually add reagent using a smaller container or pipet, not by pouring straight from the stock bottle.


The piece of glassware that would be the best choice to make a 50.0 mL solution
Glass Waste
Solid Chemical Waste
Liquid Chemical Waste
Trash Can
Sink
50 mL Beaker
50 mL Graduated Cylinder
10 mL Graduated Pipet
10 mL Graduated Cylinder
50 mL Volumetric Flask
Answer: 50 mL Volumetric Flask
Explanation:
You need to prepare a solution with an exact final volume of 50.0 mL.
50 mL Beaker
Not precise, volume markings are a rough estimate.
Graduated Cylinders
Decent for measuring, but not as accurate as a volumetric flask for making a solution to an exact final volume.
10 mL Graduated Pipet
Used to transfer small measured amounts, not to set a final total volume.
50 mL Volumetric Flask
Calibrated to contain one precise volume (50.0 mL) when filled to the line.

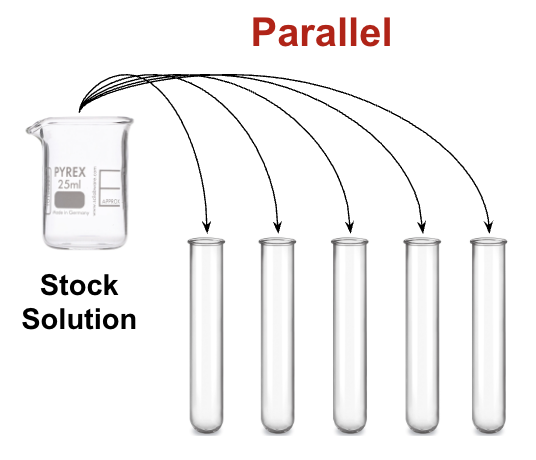
The best choice of glassware to use when measuring solutions for parallel dilutions.
Glass Waste
Solid Chemical Waste
Liquid Chemical Waste
Trash Can
Sink
50 mL Beaker
50 mL Graduated Cylinder
10 mL Graduated Pipet
10 mL Graduated Cylinder
50 mL Volumetric Flask
Answer: 10 mL Graduated Pipet
Explanation:
Parallel Dilutions = Making several diluted solutions side by side, usually for something like a standard curve. The key is that each dilution needs accurate, repeatable volumes.
For dilutions, small differences in volume change the concentration a lot. So you want the tool that gives the most precise measurement of liquid volume.


The best choice of glassware to measure the volume of NaOH that you added to your solution in the copper cycle.
Glass Waste
Solid Chemical Waste
Liquid Chemical Waste
Trash Can
Sink
50 mL Beaker
50 mL Graduated Cylinder
10 mL Graduated Pipet
10 mL Graduated Cylinder
50 mL Volumetric Flask
Answer: 10 mL Graduated Cylinder
Explanation:
Add approximately 5 mL of Sodium hydroxide (NaOH)
All volumes were “approximately” measured, not “precisely” measured.

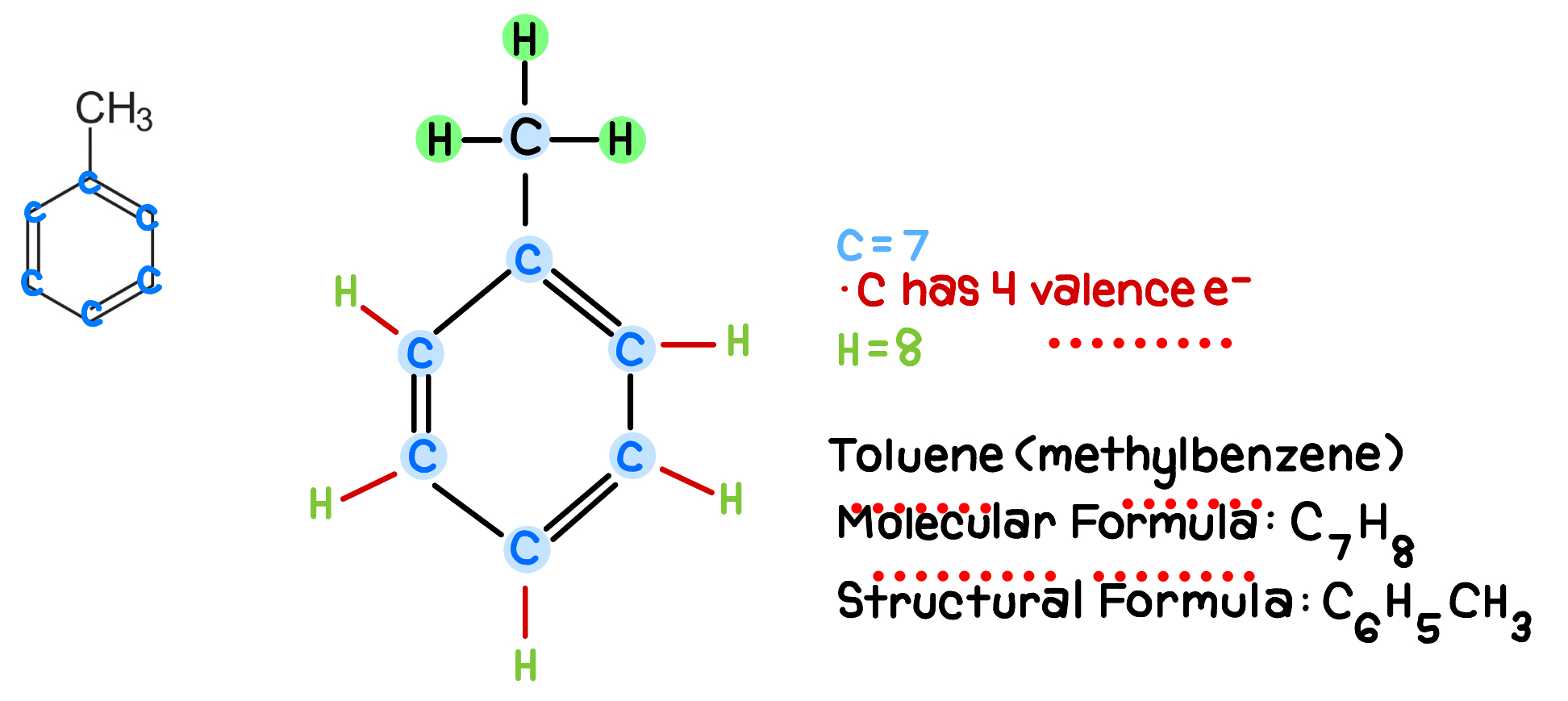
For this molecule, select all statements below that apply:
Forms no hydrogen bonds
Forms hydrogen bonds with itself.
Forms hydrogen bonds with water.
Answer: Forms no hydrogen bonds
Explanation:
Hydrogen bonding needs:
Hydrogen bond donor: H atom attached to a N, O, or F.
Hydrogen Bond Acceptor: Often another N, O, or F.
Check if this molecule has donors.
Toluene has only C and H.
There is no O-H, N-H, or F-H bond.
So, it can’t donate hydrogen bonds.
Check if this molecule has acceptors.
There are no n,
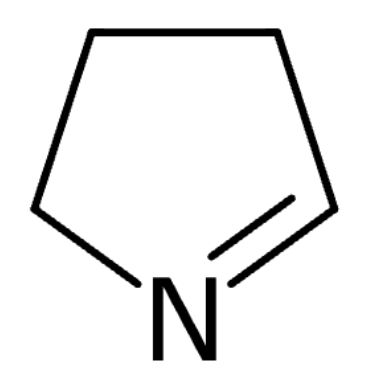
For this molecule, select all statements below that apply:
Forms no hydrogen bonds
Forms hydrogen bonds with itself.
Forms hydrogen bonds with water.
Answer: Forms hydrogen bonds with water
Explanation:
____
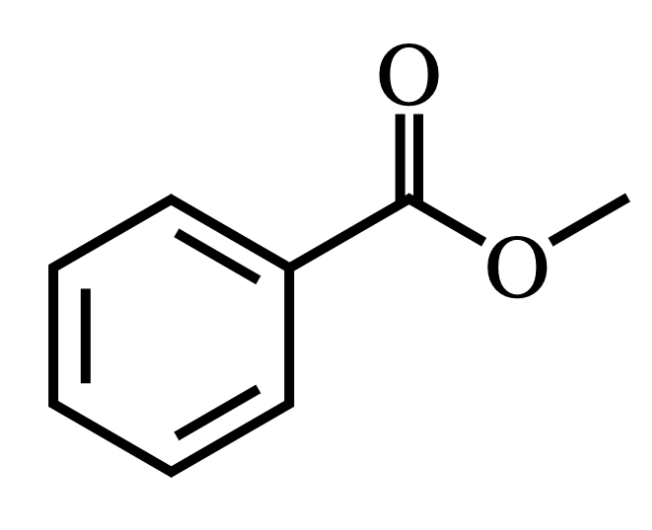
For this molecule, select all statements below that apply:
Forms no hydrogen bonds
Forms hydrogen bonds with itself.
Forms hydrogen bonds with water.
Answer: Forms hydrogen bonds with water
Explanation:
____
Why was the discovery of the first synthetic polymer revolutionary? Select all that apply.
Humans could create new materials.
It helped the environment.
Material wealth became more widespread.
Humans would need to continue to depend on natural resources.
Human manufacturing will still be constrained by the limits of nature.
Answer(s):
Humans could create new material.
It helped the environment.
Material wealth became more widespread.
Explanation:
____
What are the two ways that monomers can be joined to form polymers?
Addition
Condensation
Substitution
Formation
Answer(s):
Addition
Condensation
Explanation:
____
Can both types of polymers be melted and reformed (recycled)?
Yes, both are thermoplastic and can be easily processed, reprocessed, or recycled.
No, addition polymers are thermoplastic but condensation polymers are thermoset and cannot be melted and reformed.
No, condensation polymers are thermoplastic but addition polymers are thermoset and cannot be melted and reformed.
Answer: No, addition polymers are thermoplastic but condensation polymers are thermoset and cannot be melted and reformed.
Explanation:
____
Ammonia can be synthesized by the following reaction:
2NO (g) + 5 H2 (g) → 2NH3 (g) + 2 H2O (g)
If you begin with 87 grams of NO and 27.6 grams of H2, what is the theoretical yield of NH3? (write your answer to 1 decimal place)?
Ammonia can be synthesized by the following reaction:
2NO (g) + 5 H2 (g) → 2NH3 (g) + 2 H2O (g)
If you begin with 85.6 grams of NO and 24.5 grams of H2, what is the theoretical yield of NH3? (write your answer to 1 decimal place)?
You perform the reaction to make ammonia. What information to you need to record in order to calculate the % yield for the reaction?
Amount of NH3 produced.
Amount of water produced.
Amount of NO remaining after the reaction.
Amount of H2 remaining after the reaction.
Answer: Amount of NH3 produced.
Explanation:
____
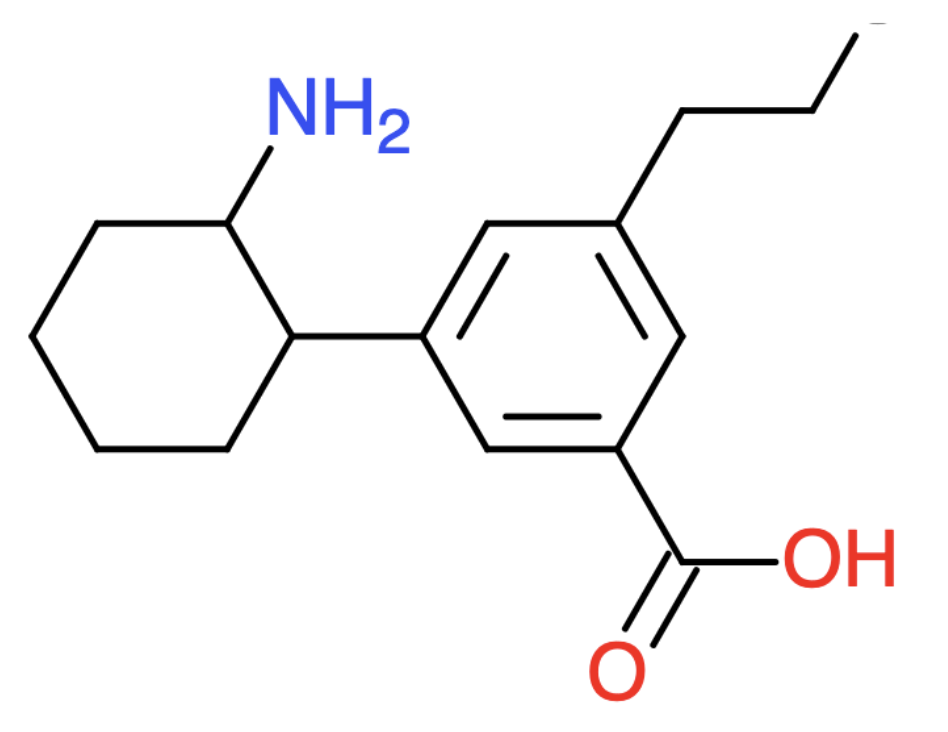
What is the formula for the molecule shown below?
C:
H:
N:
O:
Answer:
C: 16
H: 23
N: 1
O: 2
Explanation:
____
What types of impurities could be present in a synthesis?
Solvent
Unreacted starting material
Side products
Main Products
Answer(s):
Solvent
Unreacted starting material
Side products
Explanation:
____
The type of reaction when two soluble ions react to form an insoluble solid.
Answer: precipitation reaction
Explanation:
____
In the reaction Zn + H2SO4 → ZnSO4 + H2, which element, if any, is oxidized?
zinc
hydrogen
oxygen
sulfur
none of these
Answer: zinc
Explanation:
____
After heating your beaker of Cu(OH)2, you formed copper (II) oxide. Before you added the H2SO4, you were instructed to decant the supernatant, add warm water, and decant again. What was the purpose of this step?
Remove excess H2SO4
Clean off the Cu(OH)2
Clean off the CuO
Remove excess NaOH
Answer: Remove excess NaOH
Explanation:
____
What are the things to remember or use when writing a procedure?
passive voice
paragraph form
past tense
present tense
first person
bulleted list form
Answer(s):
passive voice
paragraph form
past tense
Explanation:
____
2AgNO3(aq) + CaCl2(aq) --> 2AgCl(s) + Ca(NO3)2(aq)
You have a 1.00 M solution of silver nitrate and a 0.100 M solution of calcium chloride. Following the above reaction, you react 25.00 mL of each solution. What is the theoretical yield of solid? (Note: MW of AgCl = 143.32 g/mol)
Answer: 0.717
Explanation:
____
Error analysis would be found in which part of your report?
Discussion
Procedure
Results
Data
Answer: Discussion
Explanation:
____
During the aspirin synthesis experiment, you isolated the aspirin with what equipment?
Buchner funnel
Gravity funnel
Separatory funnel
Erlenmeyer flask
Answer: Buchner funnel
Explanation:
____
Ethanol has a lower viscosity than honey.
True
False
Answer: True
Explanation:
____
In which state of the following compounds does nitrogen have the most positive oxidation state?
NaNO2
N2O
HNO3
NO2
NH4Cl
Answer: HNO3
Explanation:
____
Chemicals that you scrap off of your filter paper would be placed in the ___________.
solid chemical waste
liquid chemical waste
trash can
glass waste
Answer: solid chemical waste
Explanation:
____
The repeating part or sub-unit of a polymer.
Answer: monomer
Explanation:
____
In addition to avoiding spills, recapping chemical bottles and jars also prevents this (select the BEST answer).
contamination
evaporation
reactions
confusion
Answer: contamination
Explanation:
____
You need 50 mL of a 1.0 M NaCl solution. After you correctly measure the mass of the NaCl needed, you add the salt to 50.00 mL of water and it dissolves. Your solution is __________.
Too dilute
Too concentrated
Correct
Answer: Too dilute
Explanation:
____
Color and appearance are examples of ___________ observations.
qualitative
quantative
Answer: qualitative
Explanation:
____
When evaluating the aspirin product, we calculated the _____ and the _____ .
Answer(s):
percent yield
percent purity
Explanation:
____
During the semester, we often separated solid chemicals from liquid chemicals. Which technique was not used?
decanting
diltration
centrifugation
evaporation
Answer: evaporation
Explanation:
____
When considering the strength of polymers such as nylon, stronger polymers had ________ carbon chains.
Shorter
Longer
Answer: Shorter
Explanation:
____
In the copper cycle, if we wrote reactions in the _______ form, we would show the species as ions and include the spectator ions.
Answer: ionic equation
Explanation:
____