Ch. 24 - Lipid and Amino Acid Metabolism
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
Lipid Metabolism
During digestion:
Triglycerides are hydrolyzed to glycerol, fatty acids, and monoglycerides
Phosphoglycerides are hydrolyzed to their component substances
Triglycerides and phosphoglycerides are resynthesized in…
the cells of intestinal mucosa
Chylomicron
are lipoproteins formed from the combination of insoluble lipids and proteins
made for the transport of insoluble lipids within the lymph and blood
Modified by the liver into smaller lipoprotein particles
Behavior of Blood and Plasma Lipids
Behavior of blood lipids parallels the behavior of blood sugar
Concentration of Blood Lipids and Blood Sugar
Increases after a meal
Returns to normal as a result of storage in fat depots and oxidation to provide energy
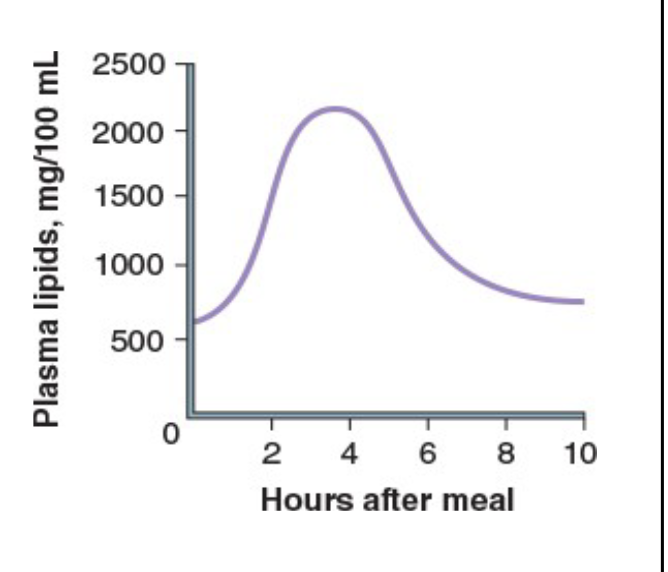
Concentration of Plasma Lipids:
Rises within 2 hours of a meal
peaks in 4–6 hours
Drops rapidly to a normal level
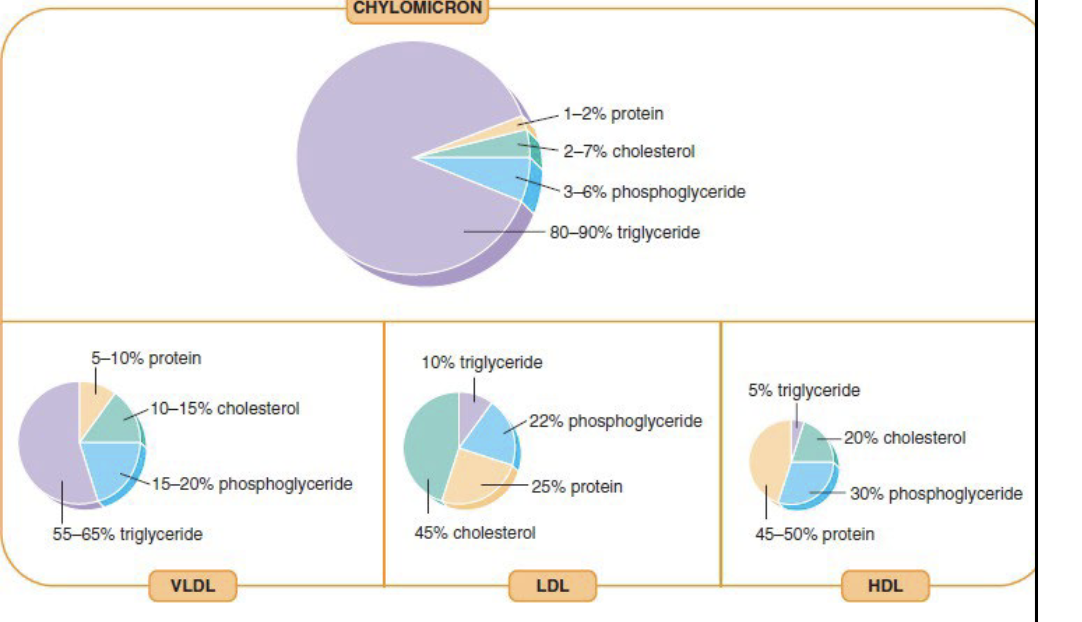
Classification of Lipoproteins
Based on density
Lipids are less dense than proteins
Higher the lipid concentration of a lipoprotein, the lower the density
VLDL - Very low density lipoprotein
LDL - Low density lipoprotein
HDL - High density lipoprotein
Energy from Fat Mobilization
When the body’s stores of glycogen are depleted, fatty acids are used as energy sources
Helps conserve glycogen storage and glucose for:
Brain cells, which cannot directly obtain nutrients from the blood because of the blood-brain barrier
RBCs, which do not have mitochondria and therefore cannot oxidize fatty acids
When body cells need fatty acids for energy, the endocrine system produces hormones that interact with adipose tissue
Fat Mobilization
Hydrolysis of stored triglycerides, followed by the entry of fatty acids and glycerol into the bloodstream
In blood, mobilized fatty acids form a lipoprotein with the plasma protein called serum albumin and are transported to tissue cells in this form
Is glycerol water soluble or insoluble?
Glycerol is water-soluble
Dissolves in blood and is transported to cells that need it
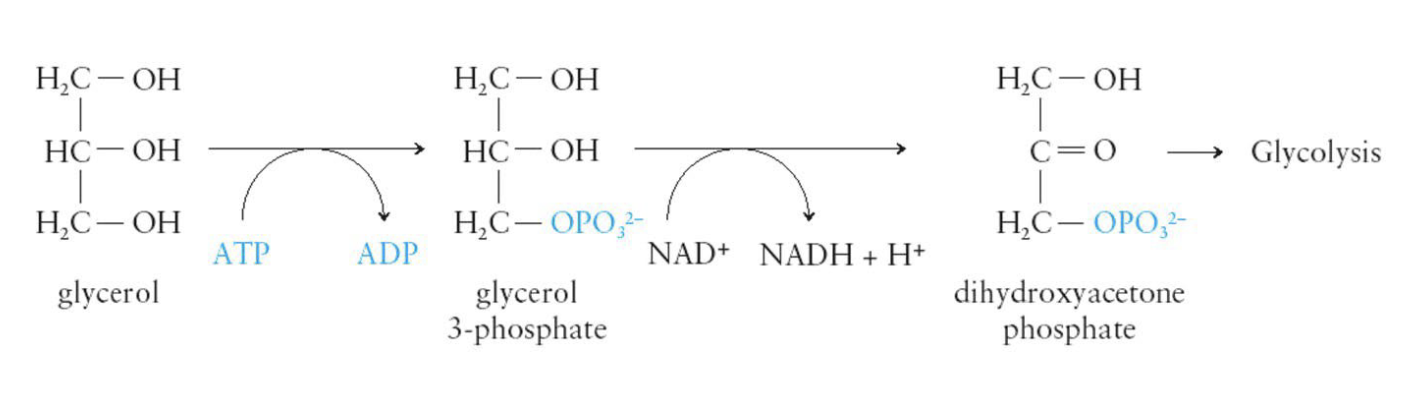
Glycerol Metabolism
Glycerol is converted in the cytoplasm to dihydroxyacetone phosphate, a chemical needed for glycolysis
By entering glycolysis, glycerol can be converted to pyruvate and can help in cellular energy production
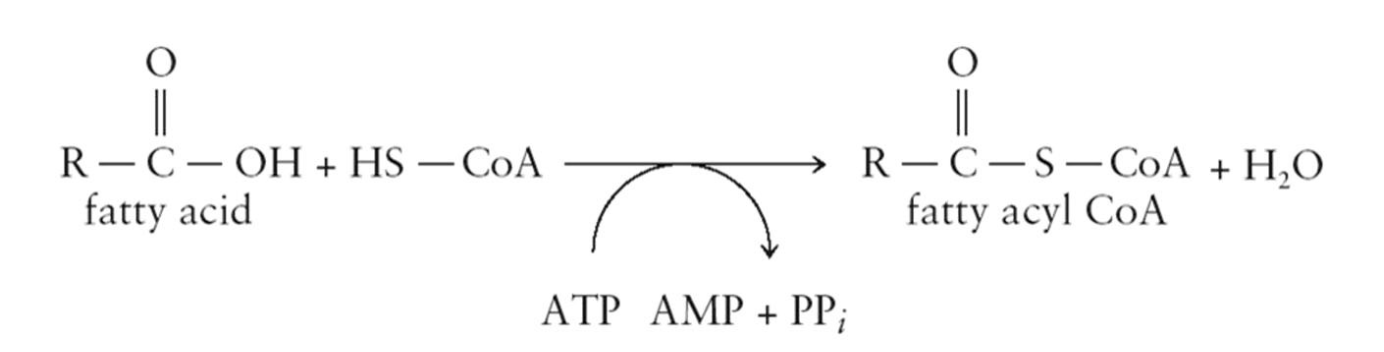
Oxidation of Fatty Acids: Step 1
Before fatty acids can be catabolized, they must be activated
Activation involves converting from fatty acid to fatty acyl CoA
catalyzed by acyl CoA synthetase
Energy is provided by the hydrolysis of ATP to AMP and PPi and the hydrolysis of PPi to 2Pi
Oxidation of Fatty Acids: Step 2
After activation, the fatty acyl CoA molecules that enter the mitochondria are degraded in the fatty acid spiral by β-oxidation
the second (beta) carbon is oxidized to a ketone
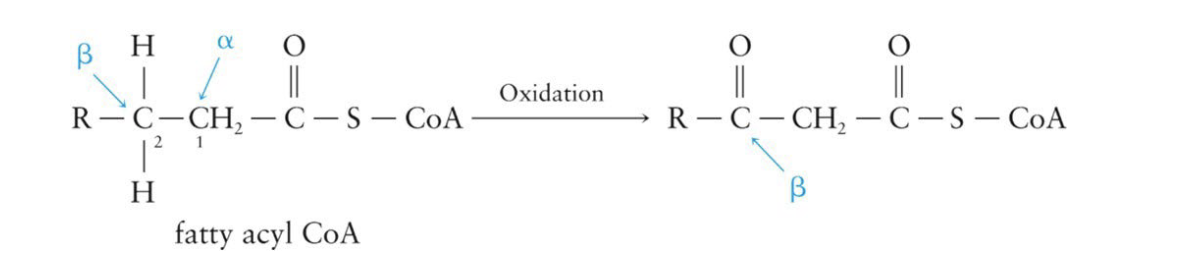
Oxidation of Fatty Acids: Step 3
then, the chain is broken between the α- and β-carbons by the reaction with coenzyme A
produces a new fatty acyl CoA and an acetyl CoA
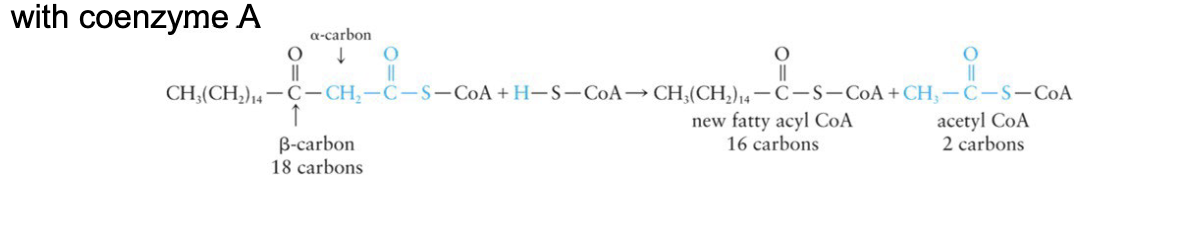
Oxidation of Fatty Acids: Step 4
New fatty acyl compound enters the β-oxidation process at step 1
Sequence is repeated until the fatty acyl CoA is completely degraded to acetyl CoA
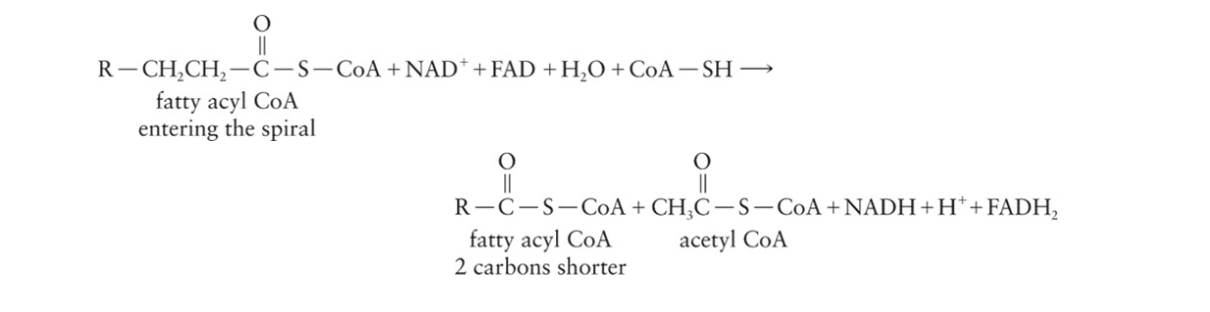
β-oxidation pathway is called the fatty acid spiral
Each pass through the fatty acid spiral reduces the fatty acyl CoA by 2 carbons and produces one molecule each of acetyl CoA, NADH, and FADH 2
Breakdown of Stearic Acid
Requires eight passes through the β-oxidation sequence
Produces:
9 molecules of acetyl CoA
8 molecules of FADH2
8 molecules of NADH
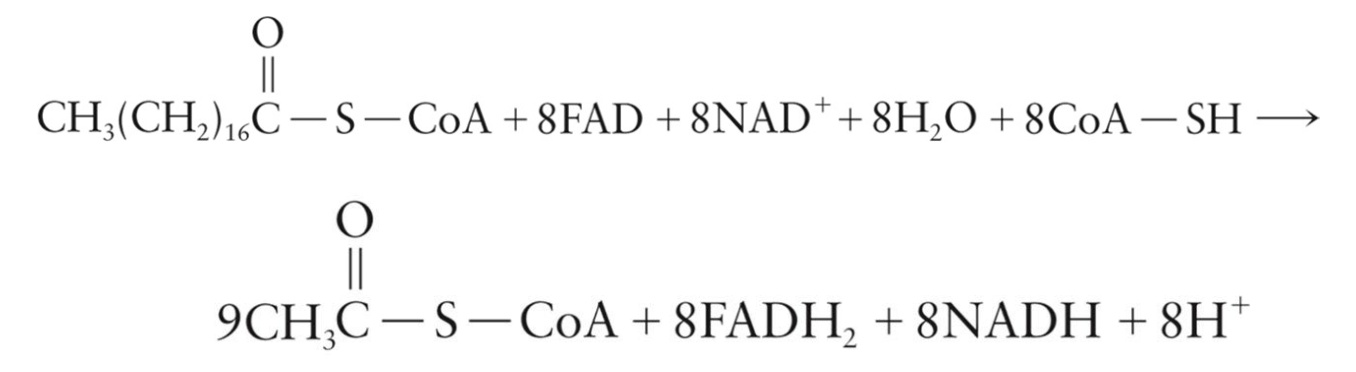
Energy from Fatty Acids
Energy of fatty acids is more dense than carbs
lipids are nearly 25% more efficient than carbs as energy-storage systems
lipids contain more than twice the energy of carbs
Lipids are a more reduced form of energy compared to glucose, which is partially oxidized
Ketone Bodies
Created in the liver when excess acetyl CoA is produced by fatty acid oxidation than can be processed by the citric acid cycle
During a fast, the amount of glycolysis decreases and less oxaloacetate is synthesized
Lack of oxaloacetate reduces the activity of CAC
Includes acetoacetate, β-hydroxybutyrate, and acetone
Carried by the blood to body tissues where they are oxidized to
meet energy needs
concentration of ketone bodies in the blood averages at 0.5 mg/100mL
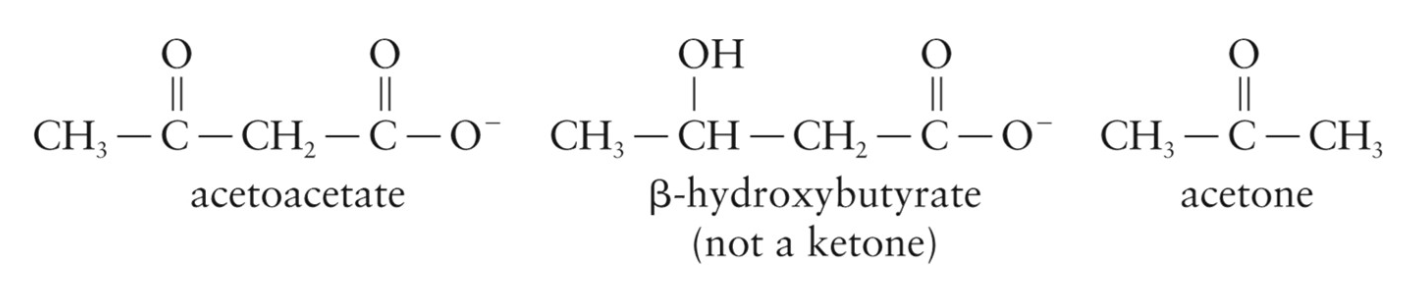
Ketonemia
Presence of elevated level of ketone bodies (higher than 20 mg/100 mL) in the blood
Ketonuria
Presence of ketone bodies in the urine
Occurs at a level of about 70 mg/100 mL when the renal threshold for ketones is exceeded and ketone bodies are excreted
Acetone Breath
Condition in which acetone can be detected in the breath
Ketosis
Condition in which ketonemia, ketonuria, and acetone breath exist together
Can lead to a condition called ketoacidosis, where blood pH lowers because of elevated levels of ketone bodies
Treatment of Conditions Related to Ketone Bodies
Diabetes-related ketosis can be treated with insulin
Insulin restores normal glucose metabolism and reduces the rate of formation of ketone bodies
Ketosis accompanied by severe dehydration is treated by administering intravenous solutions that contain sodium bicarbonate
Returns fluid and acid–base balance
Number of acetyl CoA molecules can be determined using:
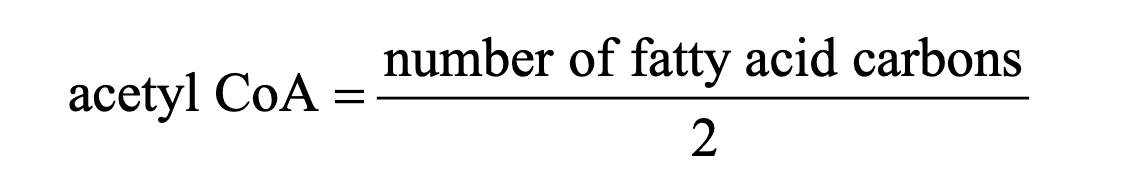
Number of trips through the fatty acid spiral can be determined using:
number of trips = molecules NADH = molecules FADH
number of trips is also one less than the number of acetyl CoA molecules
Every acetyl CoA =
10 ATP
Every NADH =
2.5 ATP
Every FADH2 =
1.5 ATP
Summary for a 10-carbon fatty acid:
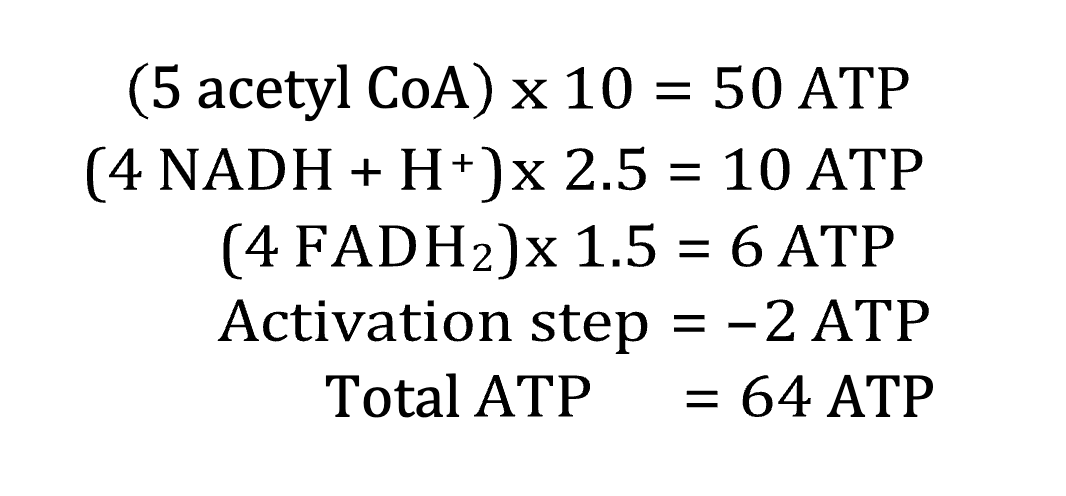
Fatty Acid Synthesis versus Fatty Acid
Degradation
These 2 pathways are not simply the reverse of each other
Degradation occurs in the mitochondria
Biosynthesis occurs in the cytoplasm
Both processes require units of 2 carbon atoms from acetyl CoA, which is made in the mitochondria and must be transported to the cytoplasm for biosynthesis
Transportation occurs by reacting acetyl CoA with oxaloacetate
acetyl CoA + oxaloacetate + H2O → citrate + CoA — SΗ
Citrate reacts to regenerate acetyl CoA and oxaloacetate once in the cytoplasm
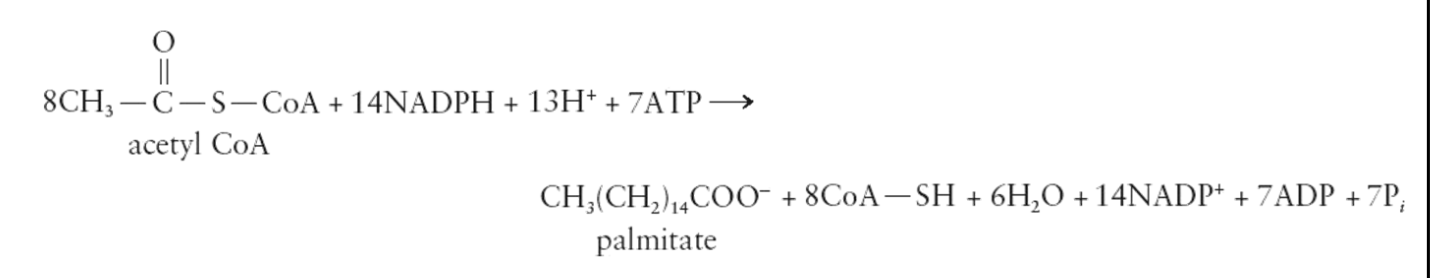
Fatty Acid Synthesis
Occurs by a series of reactions that are catalyzed by fatty acid synthetase system
Made up of six enzymes and acyl carrier protein (ACP)
After synthesis, the fatty acids are incorporated into triglycerides and stored in fat in adipose tissues
Some plants and bacteria possess enzymes to convert fats to carbs as part of their normal metabolism
Liver in Fatty Acid Synthesis
The liver modifies body fats by lengthening or shortening and saturating or unsaturating fatty acid chains
Can the human body synthesize polyunsaturated fatty acids?
No, the human body cannot synthesize polyunsaturated fatty acids
but it can convert linoleic acid and linolenic acid from the diet into other polyunsaturated fatty acids
Can the human body convert glucose to fatty acids?
Yes, it can convert glucose to fatty acids
Cannot convert fatty acids to glucose
Amino Acid Metabolism
About 75% of amino acids in normal, healthy adults are used to provide building blocks for the synthesis of proteins
Amino acid pool is continually used for the synthesis of other nitrogen-containing biomolecules
Amino acids in excess are not stored for later use, they are degraded
Amino Acid Pool
Total supply of amino acids in the body
comes from:
the digestion of food
the body’s own degraded tissue
the synthesis of some amino acids in the liver
Protein Turnover
Continuing process in which body proteins are
hydrolyzed and resynthesized
Rate is expressed as a half-life
Frequent turnover rate enables the body to:
Continually renew important molecules
Respond quickly to its own changing needs
Amino Acid Catabolism
Nitrogen of amino acids is either excreted or used to synthesize other compounds
Stages in nitrogen catabolism
Transamination
Deamination
Urea formation
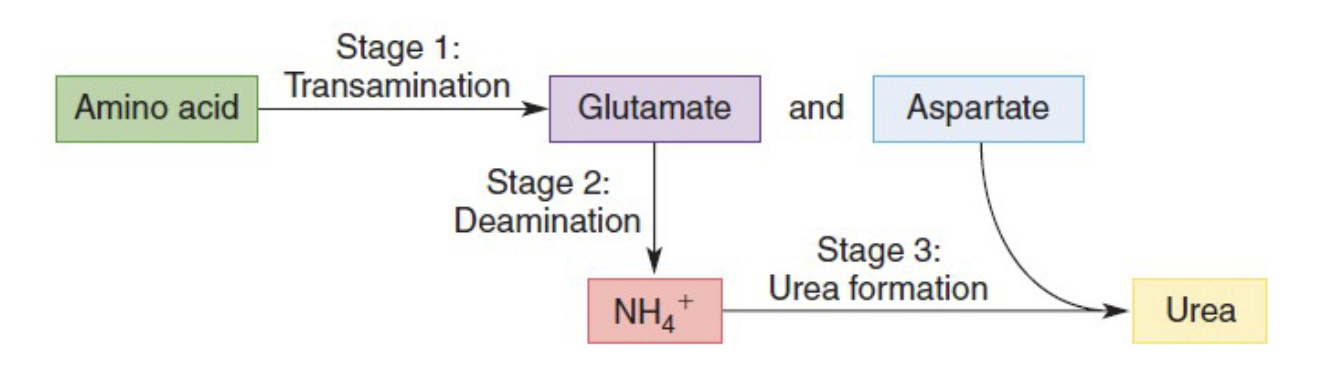
Stage 1 - Transamination
transfer of an amino group to a keto acid
Influenced by transaminases
In the transfer of amino groups to α-ketoglutarate, the carbon skeleton remains behind and is transformed into a new α-keto acid
Key reactions
Transfer of amino groups to α-ketoglutarate to form a new α-keto acid
Production of aspartate
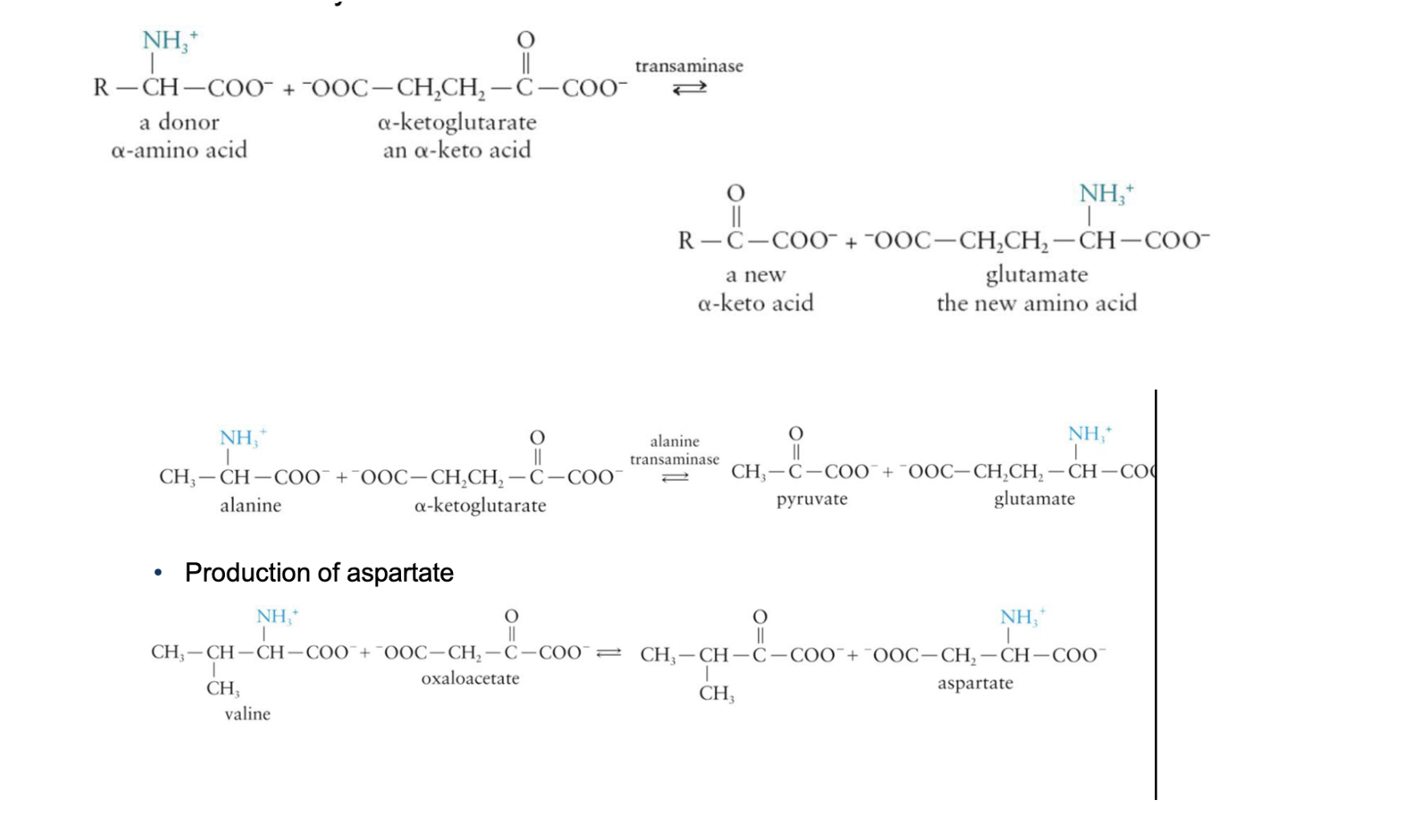
Stage 2 - Deamination
Removal of NH3+
Uses the glutamate produced in Stage 1
Glutamate dehydrogenase catalyzes the removal of the amino group from an ammonium ion and regenerates α-ketoglutarate
main source of NH4+ in humans
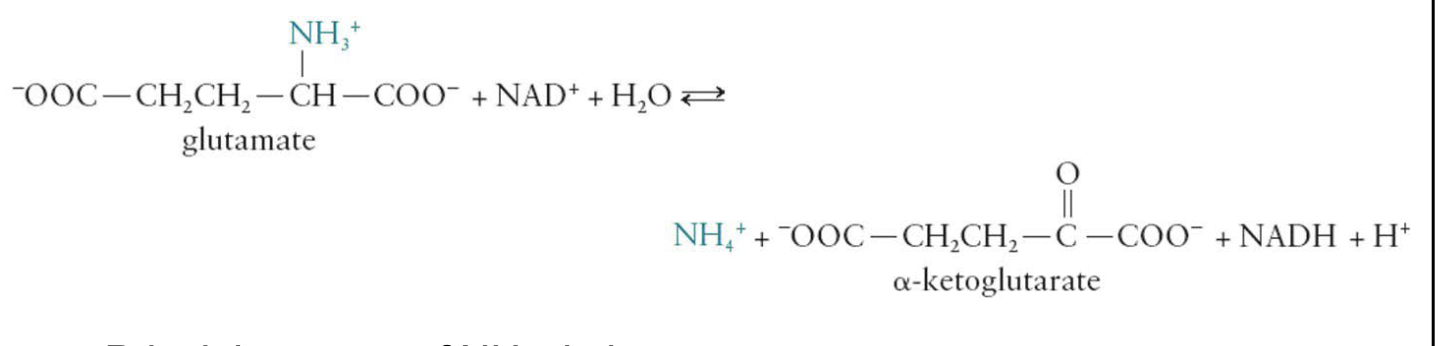
Oxidative Deamination
Reactions are catalyzed by amino acid oxidases found in the liver
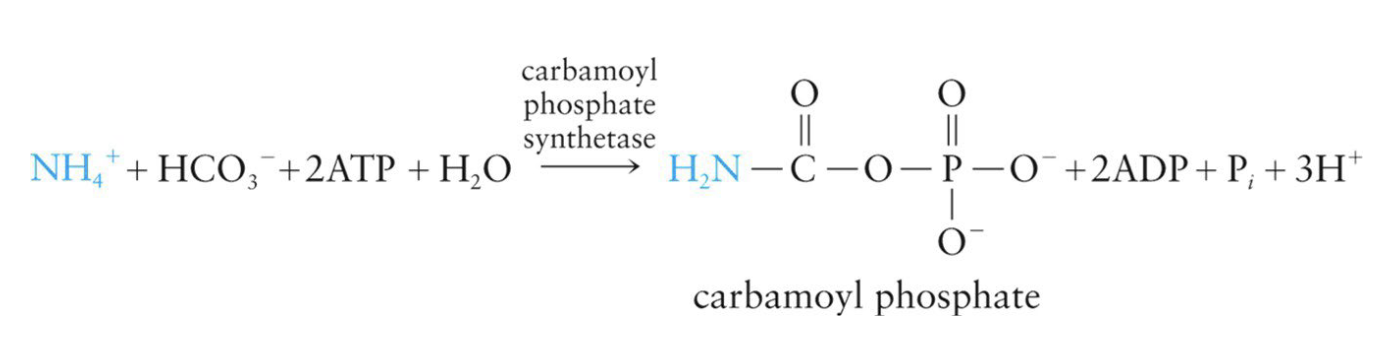
Stage 3 - Urea Formation
Urea cycle takes place
Metabolic pathway where ammonium ions are converted to urea
Processes NH4+ in the form of carbamoyl phosphate, which is made in the mitochondria from ammonium ions and bicarbonate ions
uses 2 ATP
Urea Cycle
After urea is formed, it diffuses out of liver cells into the blood, kidneys filter it out, and it is excreted in the urine
Normal urine from an adult contains 25–30 g of urea daily
Exact amount varies with protein content of the diet
Direct excretion of NH4+ accounts for a small but important amount of the total urinary nitrogen
NH4+ can be excreted with acidic ions, which help kidneys control the acid–base balance of body fluids
Fate of the Carbon Skeleton
Once a carbon skeleton is catabolized into pyruvate, it has two possible uses
Production of energy
Synthesis of glucose
Glucogenic Amino Acids
Have carbon skeletons that can be metabolically converted to materials used in glucose synthesis
Helps synthesize glucose through gluconeogenesis after glycogen stores are used up
Ketogenic Amino Acids
Have carbon skeletons that can be metabolically converted to acetyl CoA or acetoacetyl CoA
Amino Acid Biosynthesis
Nonessential amino acids can be synthesized by the body
Initiated by components of the glycolysis pathway and citric acid cycle
Glutamate, alanine, and aspartate are synthesized from α-keto acids via reactions catalyzed by transaminases
Helps adjust proportions of amino acids to meet the body’s needs by taking part in biosynthesis
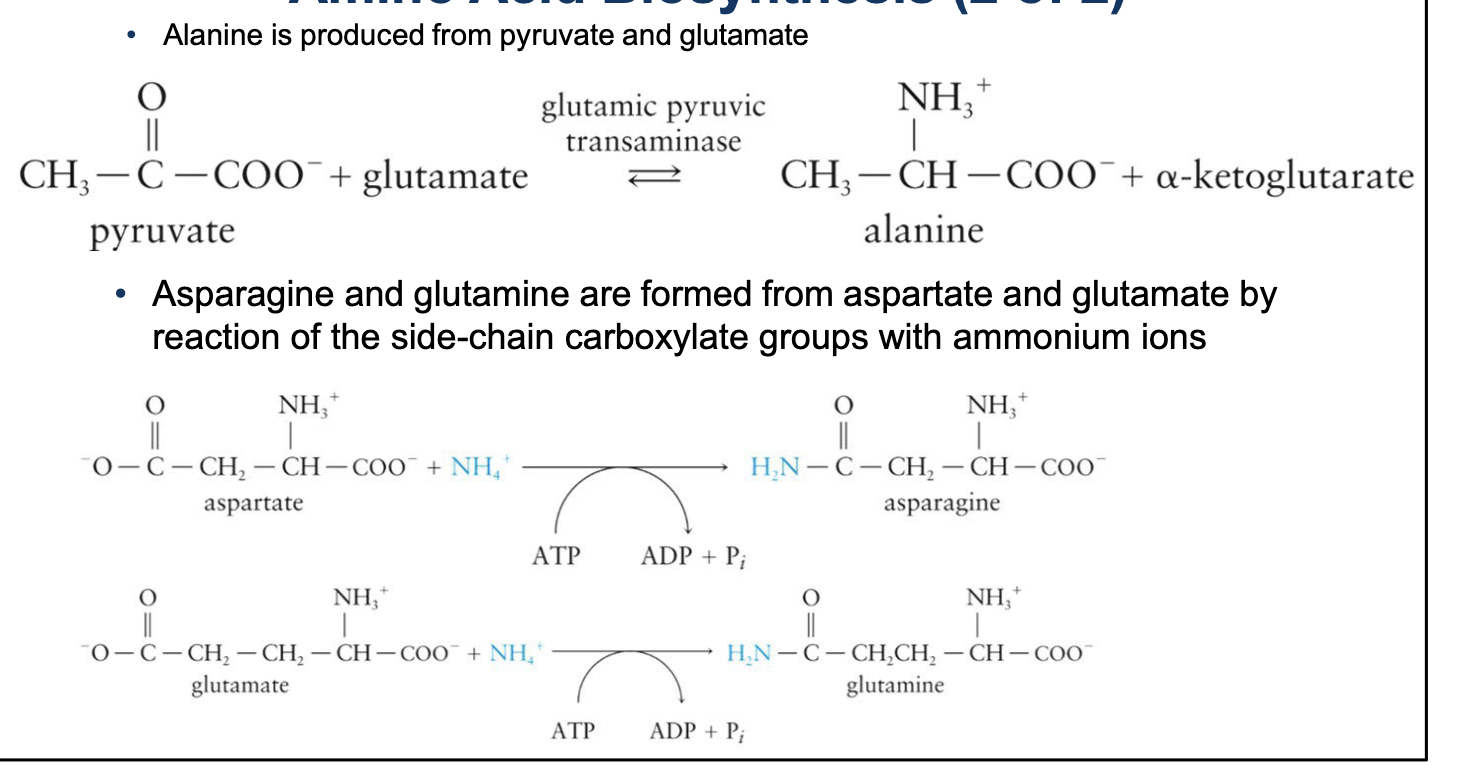
Alanine is produced from pyruvate and glutamate
Asparagine and glutamine are formed from aspartate and glutamate by reaction of the side-chain carboxylate groups with ammonium ions
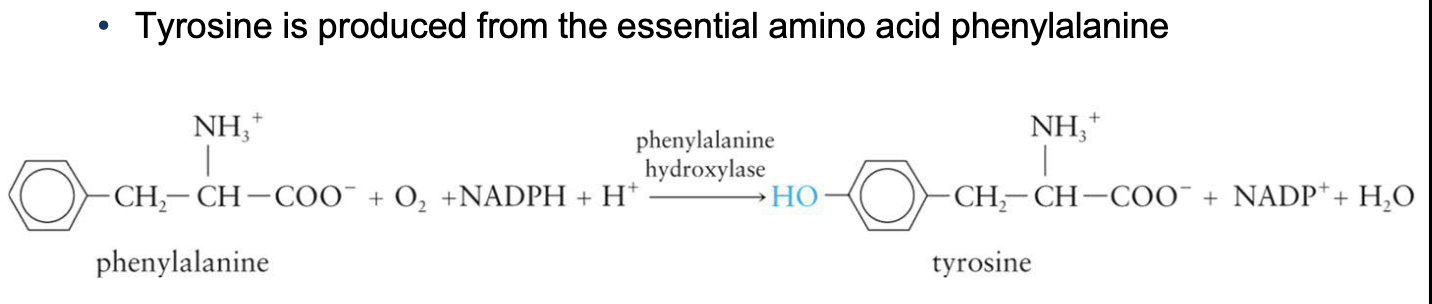
Amino Acid Biosynthesis Cont.
During digestion, triglycerides are converted to…
glycerol, fatty acids, and monoglycerides