2.3 Equilibrium
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
Define reversible reactions
Reactions that can go in both directions, i.e. reactants to products (forward reaction) then back from products to reactants (reverse reaction)
What is the extent of reaction?
How far a reaction proceeds in either the forward or reverse direction.
Define physical equilibrium
The equilibrium that exists during a physical change when the rate of the forward process is equal to the rate of the reverse process.
difference between chemical and physical changes
Physical Changes | Chemical Changes |
Involve a physical change such as evaporation | Involve the breaking of bonds between atoms |
No change in the chemical composition of the matter | Involve a change in the chemical composition of the matter |
Involve the overcoming of intermolecular forces between atoms or molecules | Involve a chemical reaction |
No new products are formed | New products are formed |
Define dynamic equilibrium
If a reversible reaction occurs in a closed system, it eventually reaches the state of dynamic equilibrium in which the rates of the forward and reverse processes are equal.
macroscopically there is no change however microscopically there is a continuous change.
the concentrations of reactants and products remain constant NOT EQUAL.
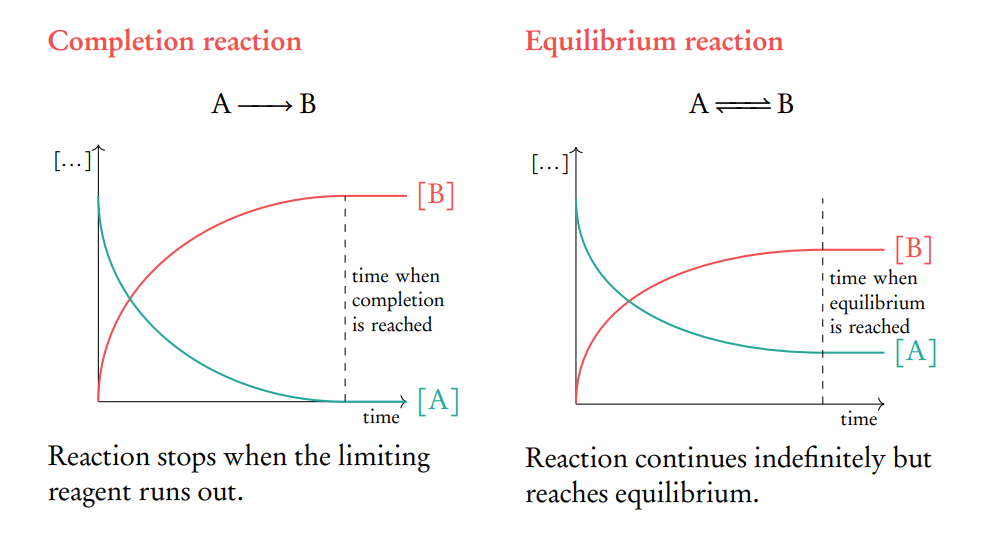
What are the characteristics of a chemical system at dynamic equilibrium?
The forward and reverse reactions proceed at equal rates.
The concentrations of reactants and products remain constant.
Macroscopic properties such as color, pressure, and density remain unchanged.
The system is in a closed environment where no matter enters or leaves.
What is the equilibrium constant (K), and how is it determined?
The equilibrium constant (K) is a ratio of the concentrations of products to reactants, each raised to the power of their stoichiometric coefficients in the balanced chemical equation.
It is determined using the equilibrium concentrations of the species involved and is temperature-dependent. A large K value indicates a reaction that favors products, while a small K value favors reactants.
What is the equilibrium law expression?
only depends on the temperature and as the concentration of reactants and products do not change at equilibrium, the reaction quotient has a very specific value.
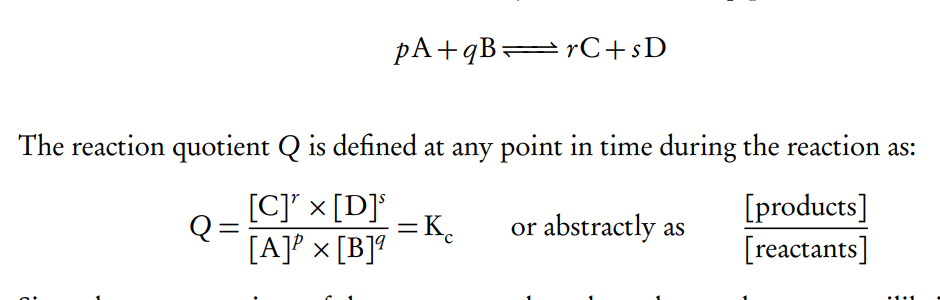
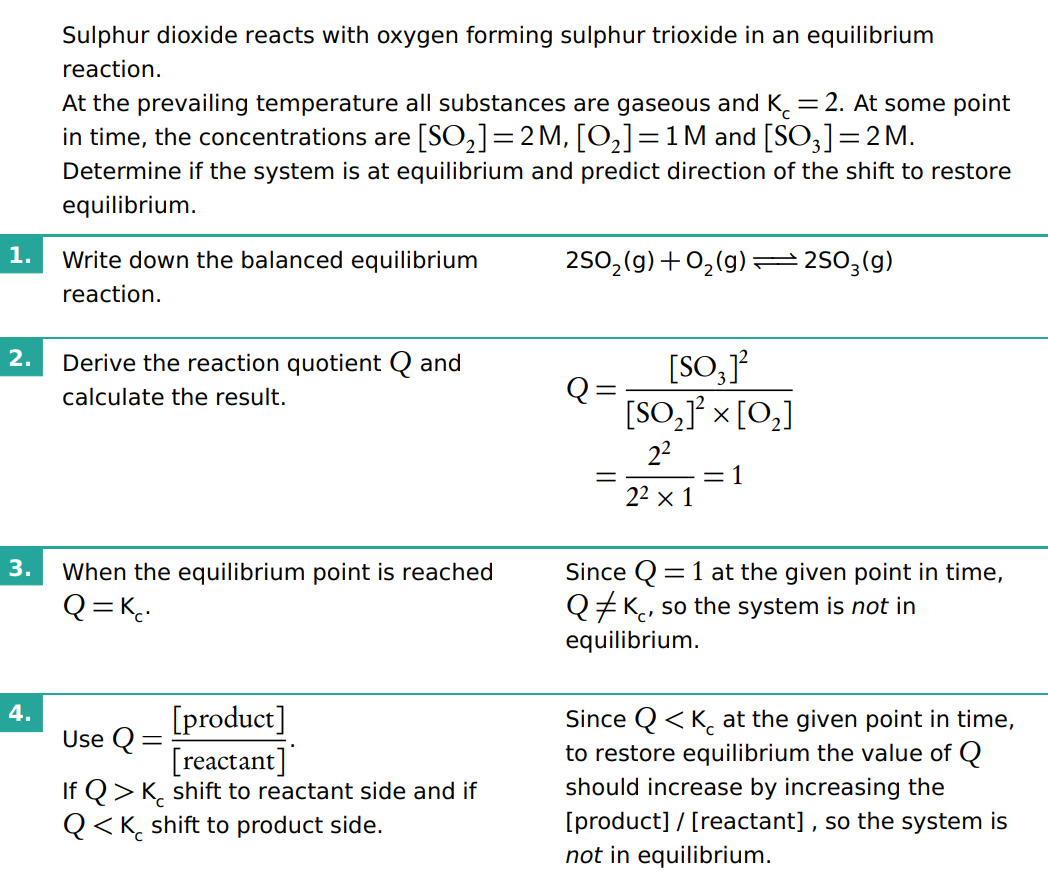
Determine if a system is at equilibrium / predict direction of shift to restore equilibrium (how to do that show and name all the step to do that)
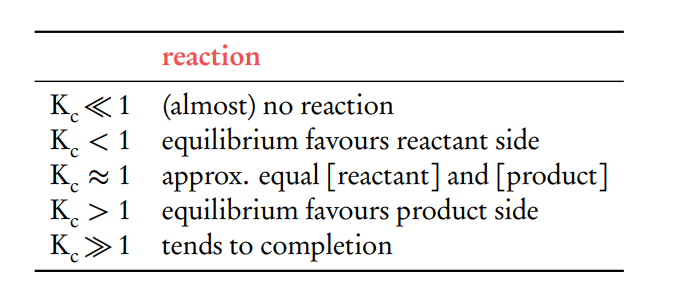
What does the magnitude of the equilibrium constant indicate about a reaction?
The magnitude of K indicates the extent of a reaction at equilibrium:
If K >> 1, the reaction strongly favors the formation of products.
If K << 1, the reaction favors the formation of reactants.
If K ≈ 1, the concentrations of reactants and products are similar, indicating a balanced equilibrium
Products over reactants .
How does a change in the concentration of reactants or products affect the position of equilibrium?
If the concentration of a reactant is increased, the equilibrium will shift towards the products to reduce the added concentration.
Conversely, if the concentration of a product is decreased, the equilibrium will shift towards the products to increase the concentration.
How does a change in pressure affect the equilibrium position in a gaseous reaction?
Changes in pressure affect reactions where gases are involved. Increasing pressure shifts the equilibrium towards the side with fewer gas molecules,
while decreasing pressure favours the side with more gas molecules.
How does temperature affect the position of equilibrium and the value of K?
Temperature changes can affect the equilibrium constant (K) and the position of equilibrium.
For exothermic reactions, increasing temperature decreases K and shifts the equilibrium towards reactants.
For endothermic reactions, increasing temperature increases K and shifts the equilibrium towards products.
What is the effect of a catalyst on the position of equilibrium?
Catalysts do not affect the position of equilibrium; they only speed up the rate at which equilibrium is achieved by lowering the activation energy for both the forward and reverse reactions.
What is the difference between heterogeneous and homogeneous equilibria?
In a homogeneous equilibrium, all reactants and products are in the same phase (e.g., all gases or all liquids).
In contrast, heterogeneous equilibrium involves reactants and products in different phases, such as a solid in equilibrium with gases.
What is Le Chateliers principle ?
Le Châtelier’s Principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to counteract the change and restore equilibrium
Explain how Le Châtelier’s Principle applies when the concentration of reactants or products in a system at equilibrium is changed.
Le Châtelier’s Principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to counteract the change and restore equilibrium.
When the concentration of a reactant or product in a system at equilibrium is changed, the system responds by shifting the equilibrium to either the right (towards the products) or the left (towards the reactants) to minimize the disturbance.
Increasing the Concentration of Reactants: I the system responds by shifting the equilibrium to the right, towards the formation of more products. This shift reduces the concentration of the added reactant as it gets converted into products, thereby partially counteracting the initial change.
Decreasing the Concentration of Reactants: the system will shift the equilibrium to the left, towards the reactants. This shift increases the concentration of the reactant by converting some of the products back into reactants, again partially counteracting the initial change.
Increasing the Concentration of Products: the equilibrium will shift to the left, favouring the formation of reactants, to reduce the concentration of the added product.
Decreasing the Concentration of Products: , the equilibrium will shift to the right, favouring the formation of more products from the reactants.
How does Le Châtelier’s Principle explain the effect of pressure changes on a system at equilibrium involving gases?
Le Châtelier’s Principle dictates that a system at equilibrium will adjust to counteract changes in external conditions, such as pressure. This adjustment is particularly significant in systems involving gases, where the pressure is dependent on the number of gas molecules present.
Increasing Pressure: (typically by decreasing the volume), the system will shift the equilibrium to the side of the reaction with fewer gas molecules.
This shift reduces the total number of gas molecules in the system, thereby lowering the pressure.
For example, in the reaction: N2(g)+3H2(g)↔2NH3(g). There are 4 moles of gas on the left side (1 mole of nitrogen and 3 moles of hydrogen) and 2 moles of gas on the right side (2 moles of ammonia). An increase in pressure will shift the equilibrium towards the right, favoring the formation of ammonia (fewer gas molecules).
Decreasing Pressure: (typically by increasing the volume), the system will shift the equilibrium towards the side with more gas molecules, increasing the number of gas molecules and thus counteracting the decrease in pressure.
No Change in Molecule Number: If the reaction involves an equal number of gas molecules on both sides of the equation, a change in pressure will not affect the equilibrium position.
Discuss the effect of temperature changes on a system at equilibrium, using Le Châtelier’s Principle.
Le Châtelier’s Principle indicates that a system at equilibrium will shift to counteract changes in temperature.
The effect of temperature on equilibrium depends on whether the reaction is exothermic (releases heat) or endothermic (absorbs heat).
Exothermic Reactions: If the temperature of the system is increased, the equilibrium will shift towards the reactants to absorb the added heat, thus reducing the temperature increase.
+ This shift reduces the amount of product formed and increases the concentration of reactants.
+Conversely, if the temperature is decreased, the equilibrium will shift towards the products, increasing the temperature by releasing more heat. For example: N2(g)+3H2(g)↔2NH3(g)+heatN_2(g) + 3H_2(g) \leftrightarrow 2NH_3(g) + \text{heat}N2(g)+3H2(g)↔2NH3(g)+heat An increase in temperature shifts the equilibrium to the left, favoring the reactants.Endothermic Reactions: In an endothermic reaction, heat is absorbed as a reactant. If the temperature of the system is increased, the equilibrium will shift towards the products, as the system absorbs the excess heat. This results in more product being formed. If the temperature is decreased, the equilibrium shifts towards the reactants to produce heat, compensating for the loss in temperature. For example: Heat+2NO2(g)↔2NO(g)+O2(g)\text{Heat} + 2NO_2(g) \leftrightarrow 2NO(g) + O_2(g)Heat+2NO2(g)↔2NO(g)+O2(g) An increase in temperature shifts the equilibrium to the right, favoring the formation of products.
Equilibrium Constant (K): The value of the equilibrium constant (K) is also temperature-dependent. For exothermic reactions, increasing the temperature decreases K, while for endothermic reactions, increasing the temperature increases K. This is because the equilibrium position shifts in response to the change in temperature.
What is the reaction quotient and how is it calculated?
The reaction quotient (Q) is a ratio that compares the concentrations of products to reactants at any point in time during a reaction.
Unlike the equilibrium constant (K), which only applies at equilibrium, Q can be used at any stage of the reaction to predict which direction the reaction will proceed to reach equilibrium.
Q is calculated using the same expression as K
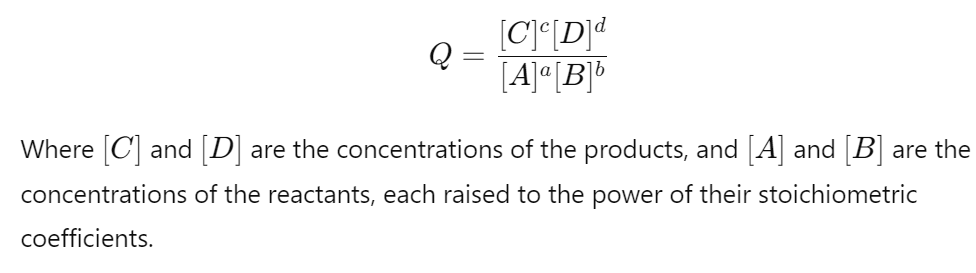
How does the value of K (equilibrium constant) relate to the value of Q (reaction quotient)
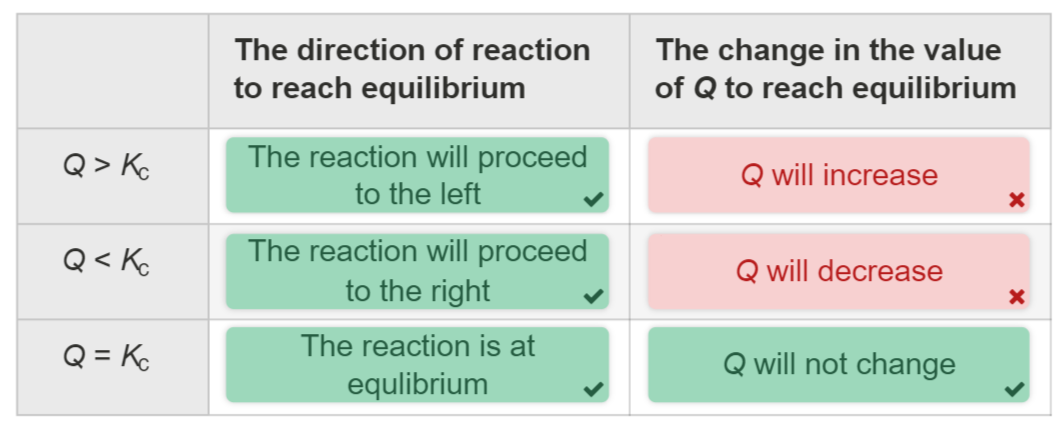
The value of Q relative to K determines the direction in which the reaction will proceed:
If Q<K, the reaction will move forward, producing more products.
If Q>K, the reaction will move in reverse, producing more reactants.
If Q=K, the reaction is at equilibrium, and no net change occurs.
When Q=K , the system is at equilibrium.
When Q≠K, the system is not at equilibrium, and the reaction will shift in the direction that brings Q closer to K
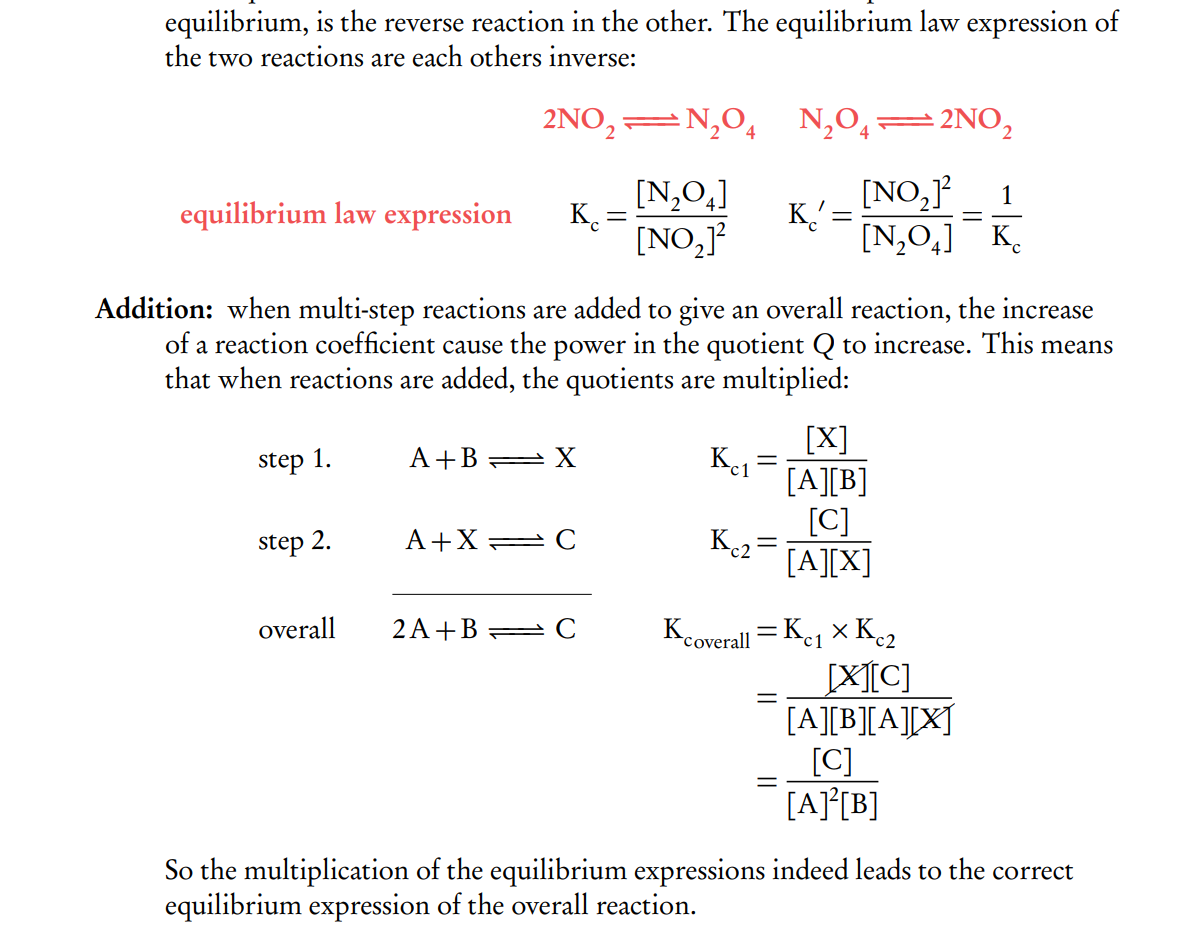
Manipulation of Kc (reversal and addition)
When an equilibrium reaction is reversed, the equilibrium constant is inversed ( 1/Kc ). And when chemical reactions are added up together their respective equilibrium constants are multiplied (Kc1 × Kc2 ).
How to calculate the reverse reaction from the forward reaction (or vice versa) use an example.
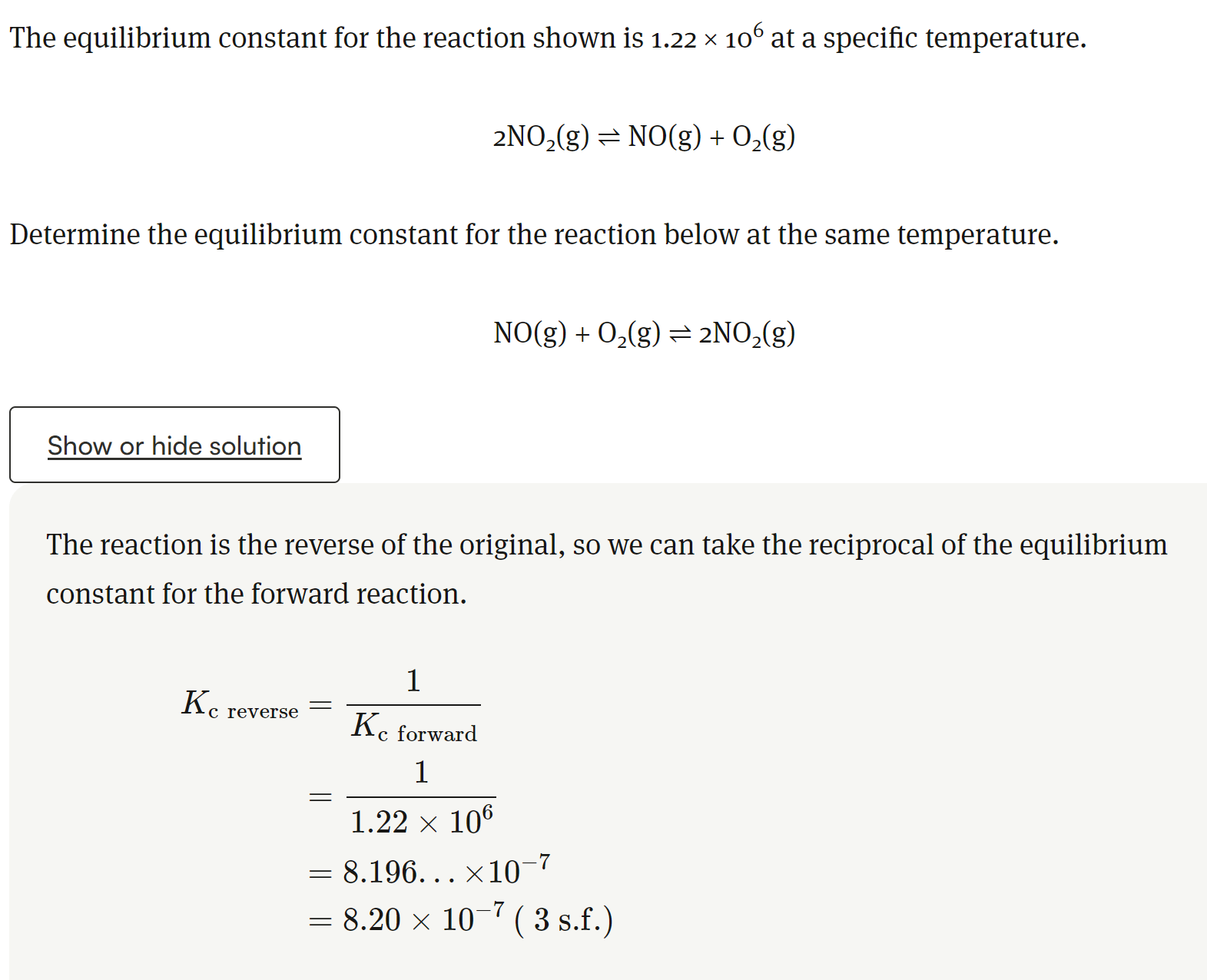
Explain the equilibriums in a liquid vapor state and a solution
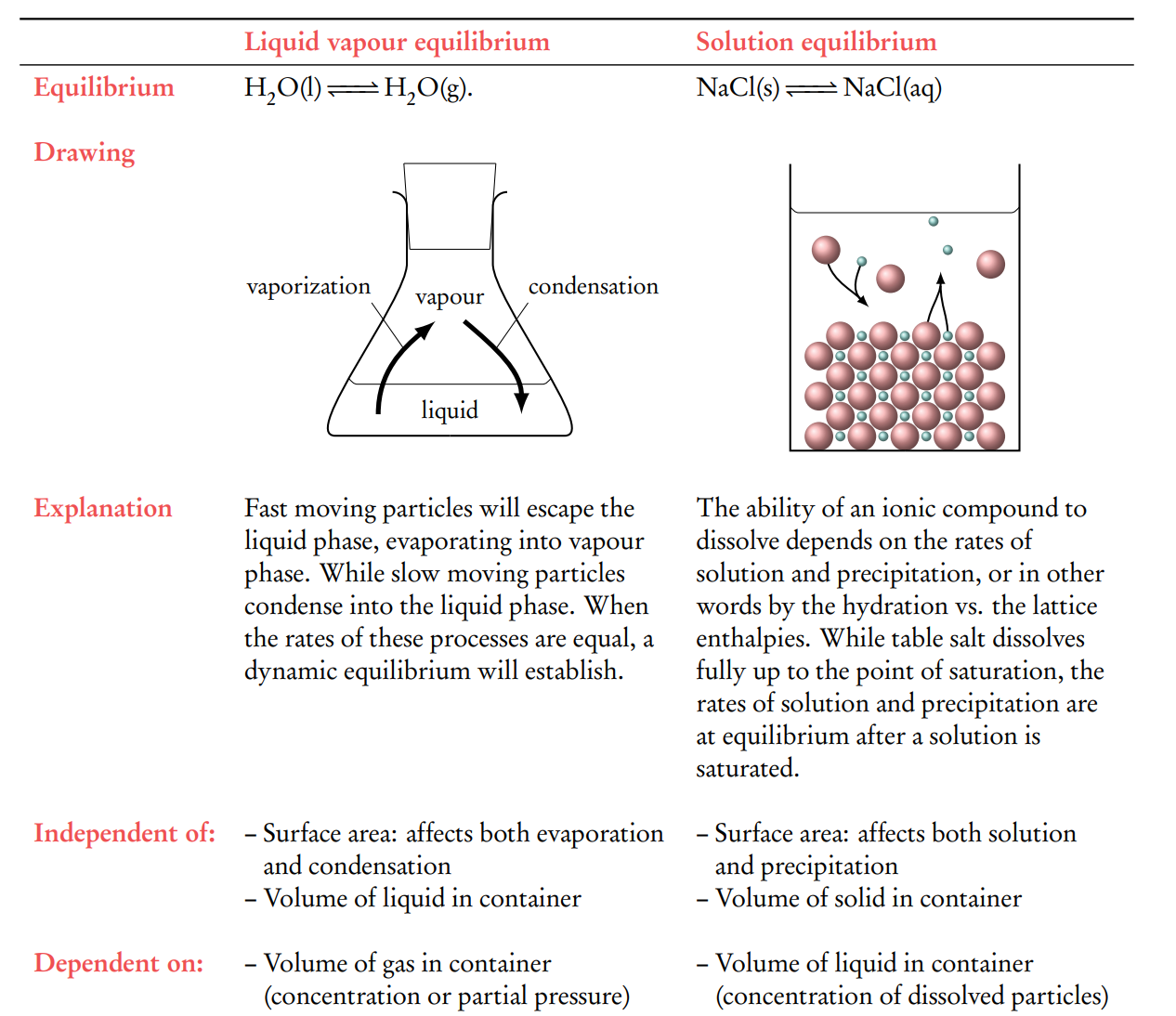
Le Chateliers principle summary
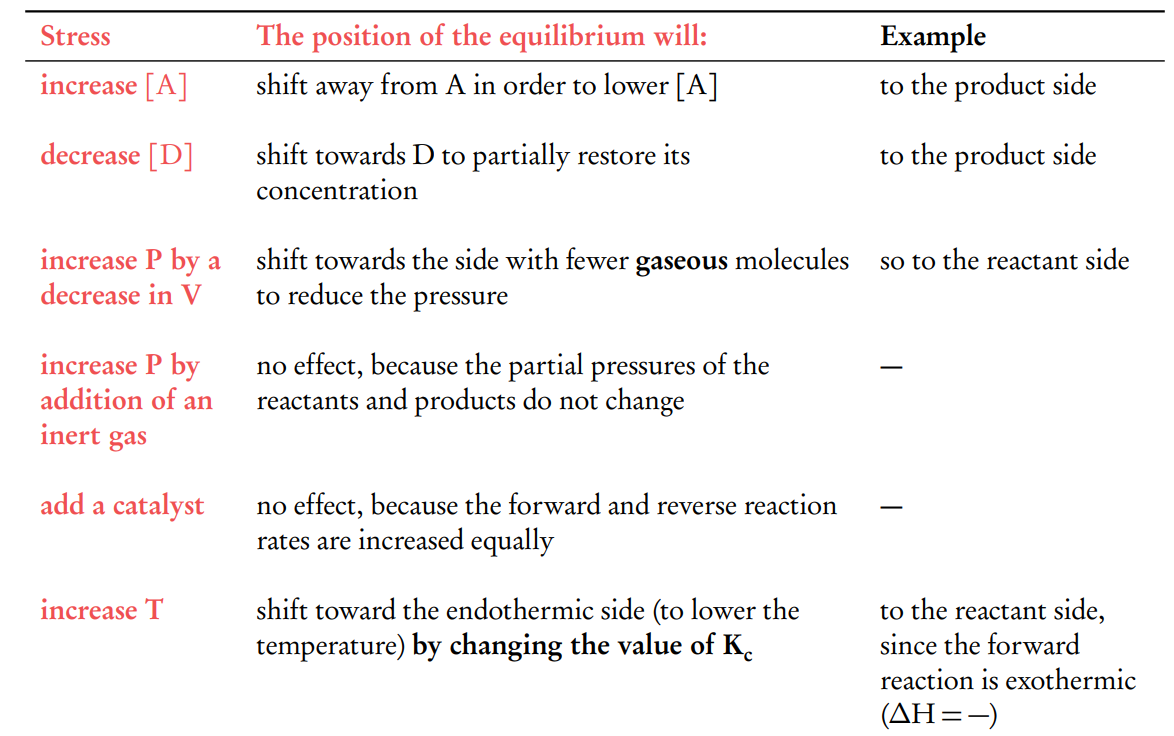
An example of an equilibrium calculation
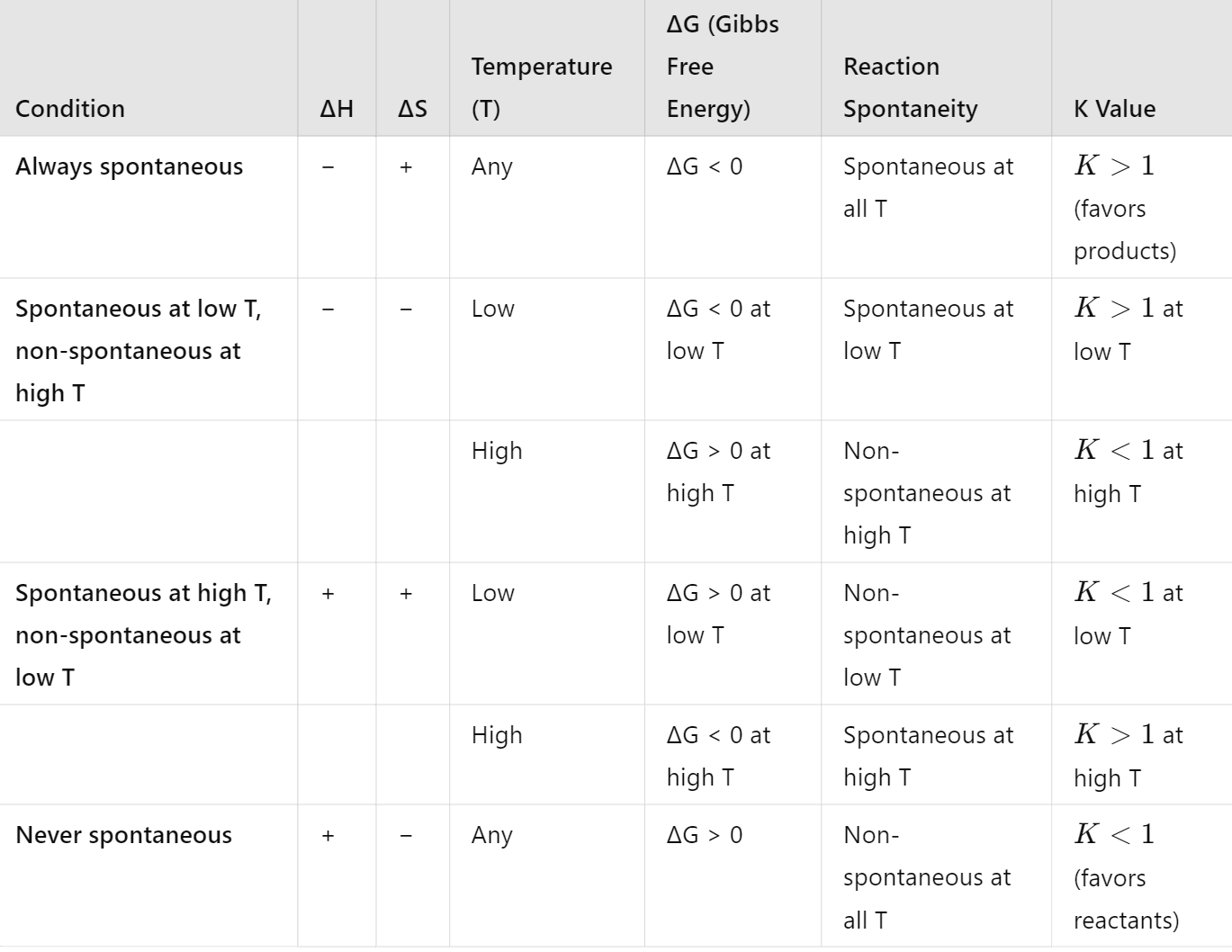
How does Gibbs free energy change (ΔG) relate to the equilibrium constant (K), and what does it indicate about the spontaneity of a reaction?
name the equations
Gibbs free energy change, ΔG, is a thermodynamic quantity that indicates the spontaneity of a reaction. It is related to the equilibrium constant K by the equation:
ΔG=ΔG∘+RTlnQ
At equilibrium, Q=K and ΔG=0 so the equation simplifies to:
ΔG∘=−RTlnK
Where:
ΔG∘ is the standard Gibbs free energy change,
R is the gas constant,
T is the temperature in Kelvin,
K is the equilibrium constant.
Interpreting ΔG and K
If ΔG∘ <0, K>1, indicates a spontaneous reaction that favors product formation at equilibrium.
If ΔG∘ >0, K<1, indicates a non-spontaneous reaction that favours reactant formation at equilibrium.
If ΔG∘=0, K=1, indicating that the reaction is at equilibrium, and neither the forward nor reverse reaction is favored.
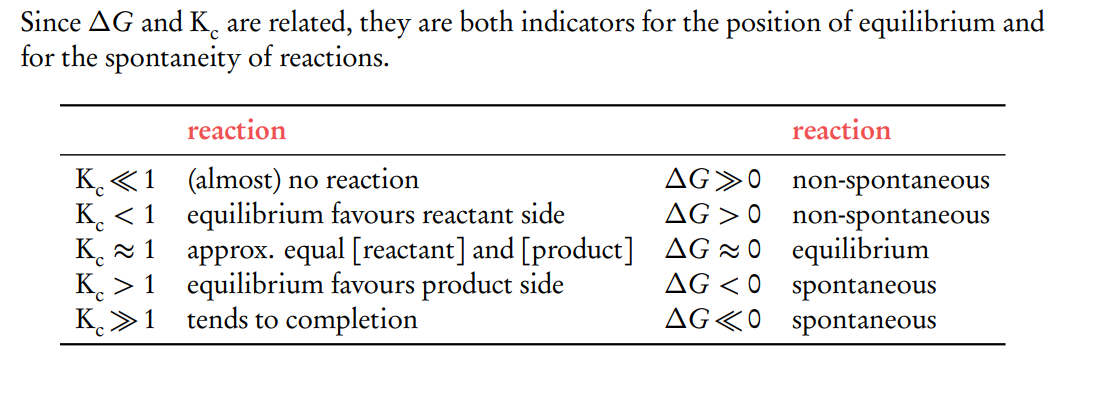
Write all the different forms and ways for the Gibbs Free Energy and Equilibrium Constant equation
a reaction at equilibrium has a minimum value of Gibbs energy and a maximum value of entropy.
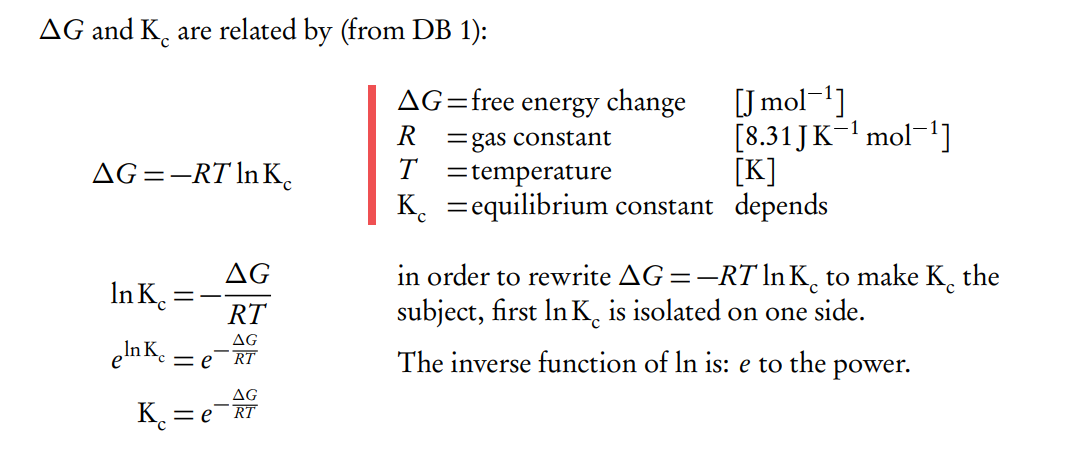
What can be inferred from the Gibbs Free Energy and Equilibrium Constant equation
From this equation we can infer that:
– the larger ∆G (positive value), the smaller Kc will to be.
– the opposite
– and since e 0 = 1, when ∆G = 0 then Kc = 1
What’s the only factor that changes the value of the equilibrium constant, Kc?
temperature
heat
increasing temperature increases the equilibrium constant.
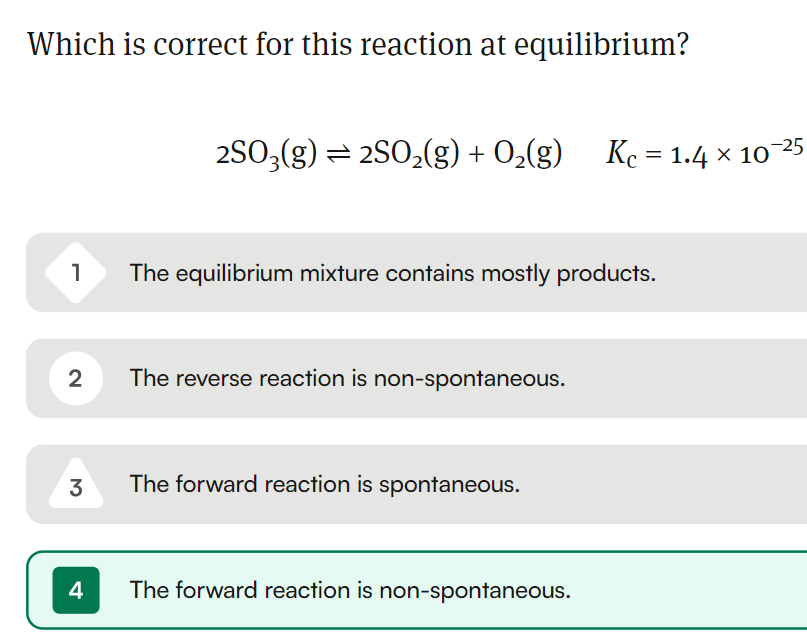
The equilibrium constant for the reaction is very small, so this means that the forward reaction is non-spontaneous and the equilibrium mixture contains mostly reactants.
The relationships between Gibbs free energy (ΔG), enthalpy change (ΔH), entropy change (ΔS), temperature, and the equilibrium constant K