Chem Cram
0.0(0)
Card Sorting
1/10
There's no tags or description
Looks like no tags are added yet.
Last updated 12:49 AM on 5/6/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
1
New cards
First Order rate
ln[A] = ln[Ao] – kt
2
New cards
Second Order rate
1/[A] = kt + 1/[Ao]
3
New cards
Zero Order Rate
[A] = [Ao] – kt
4
New cards
First Order half-life
t1⁄2 = ln(2)/k
5
New cards
Second order half-life
t1⁄2 = 1/([Ao]k)
6
New cards
zero order half-life
t1⁄2 = [Ao]/(2k)
7
New cards
Henderson Hasselboch
pH = pKa + log ([A-] / [HA])
8
New cards
Q < K
Q > K
Q < K: Rxn goes right. Right side is positive.
Q > K: Rxn goes left. Left side is positive.
9
New cards
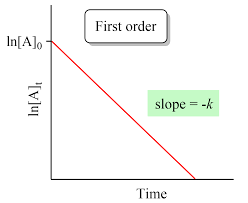
First Order Graph
10
New cards
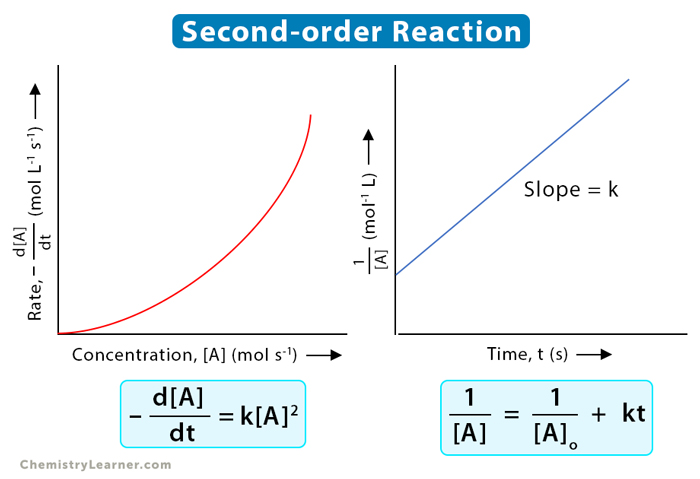
Second Order Graph
11
New cards
Zero Order Graph