Biological molecules
1/113
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
114 Terms
What do all molecules of life contain?
carbon, hydrogen, oxygen
What are the four main types of organic biological molecules?
Carbohydrates, proteins, lipids and nucleic acids
Carbohydrates
- respiratory substrates which provide energy
- structure on cell membranes + cell walls
Lipids
- source of energy
- help to insulate organisms
- waterproofing
- form membranes and hormones
Proteins
- Main component of cellular structures
- Form enzymes and chemical messengers
Nucleic acids
- Form DNA and RNA which make up genetic material
- Code for sequence of amino acids for all proteins
What are monomers?
Smaller units from which larger molecules are made
What are polymers?
Molecules made from repeating units joined together
What is a condensation reaction?
Joins two monomers together with the formation of a covalent bond and involves the elimination of a molecule of water
What is a hydrolysis reaction?
Breaks the covenant bond between two monomers and involves the use of a water molecule
What are monosaccharides?
individual sugar molecules that make up disaccharides and polysaccharides
Examples of monosaccharides
Alpha glucose, beta glucose, galactose + fructose
What are disaccharides?
formed when two monosaccharides join through a condensation reaction forming a glycosidic bond between the two OH groups
Examples of disaccharides
Maltose, sucrose + lactose
What are polysaccharides?
formed when more than two monosaccharides are joined together by a condensation reaction
What are hexose sugars?
contains six carbon atoms
What are pentose sugars?
contains five carbon atoms
What are isomers?
two or more compounds with the same formula but a different arrangement of atoms in the molecules
What are the properties of isomers like?
Different due to different arrangement of atoms
Function of alpha glucose
- Respiration
- Used to make glycogen + starch in plants
Function of beta-glucose
Building block of cellulose
Function of galactose
Used to make lactose
Function of fructose
Alternative to supply energy when there is a lack of glucose
Function of maltose
Electron donor in chemical reactions + can be broken down into glucose
What is maltose made of?
2 alpha glucose
Function of sucrose
Efficient energy transfer + increased energy store
What is sucrose made of?
Glucose + fructose
Function of lactose
Provides energy
What is lactose made of?
glucose and galactose
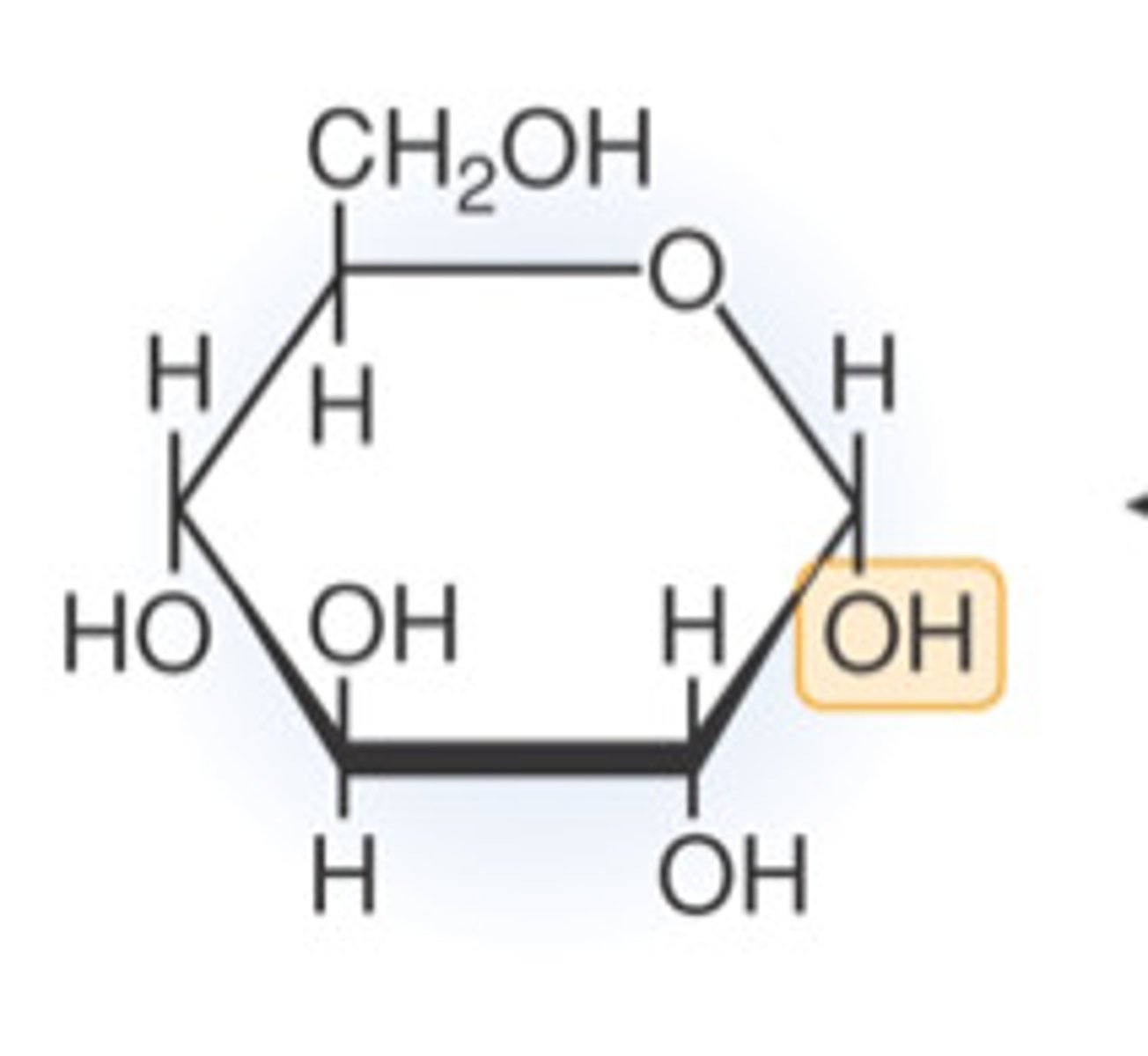
Structure of alpha glucose
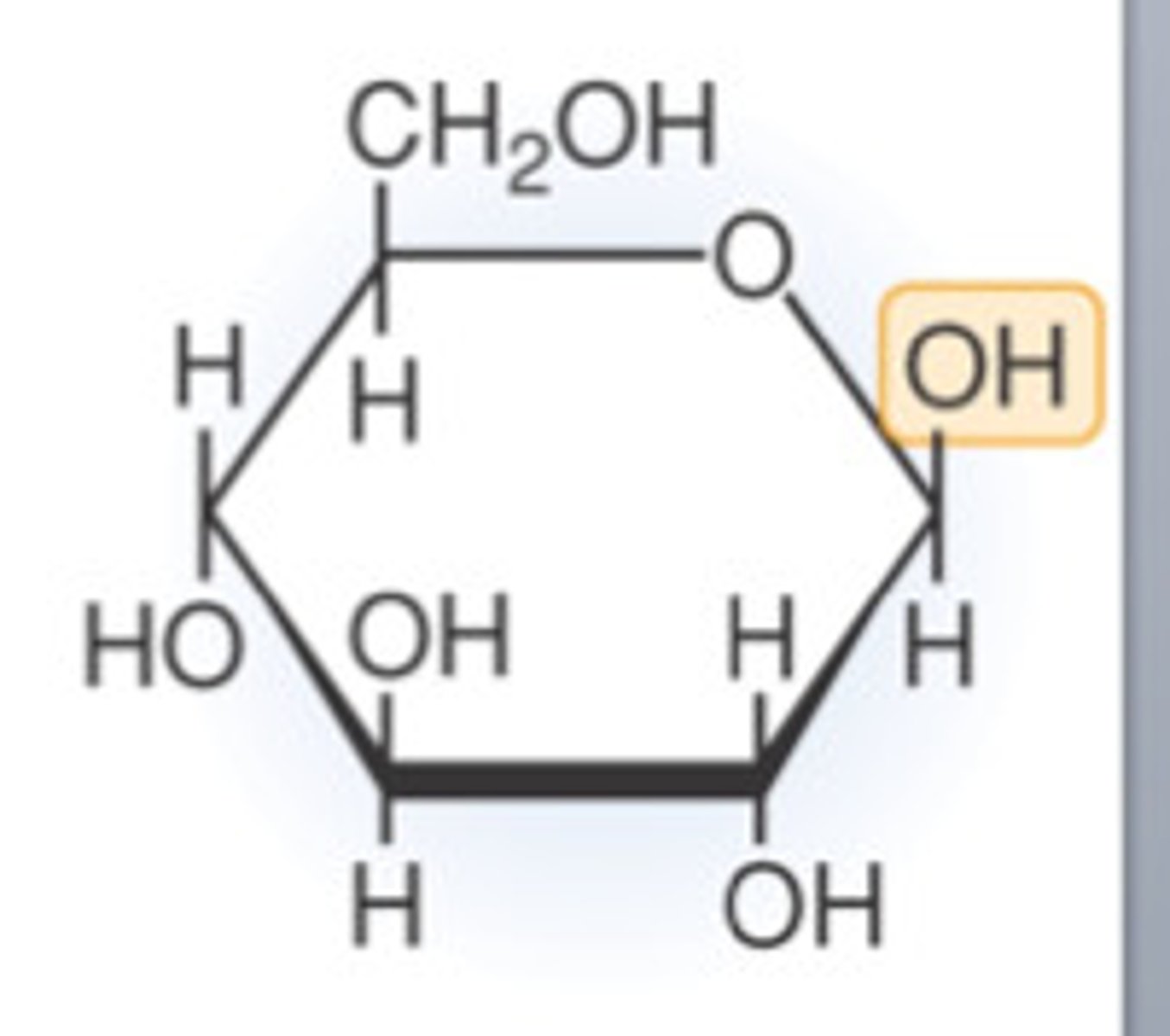
Structure of beta glucose
What are reducing sugars
All monosaccharides and some disaccharides are reducing sugars- they can lose or donate electrons to other compounds
Which disaccharide is non-reducing?
Sucrose
Test for non reducing sugars
- Add dilute HCl and heat in water bath
- Add an alkali to neutralise
- Add excess Benedict's reagent to the sample
- Heat in the water bath above 80 degrees
What is the structure of starch?
- Made of 2 types of polymers: amylose + amylopectin
Function of starch
- Store excess glucose in plants
- hydrolysed to release glucose for respiration
Structure of amylose
- Polysaccharide formed from glucose monomers joined together by 1-4 glycosidic bonds
- Long unbranched chain forming a coiled spring shape
- Coils held together by many weak hydrogen bonds
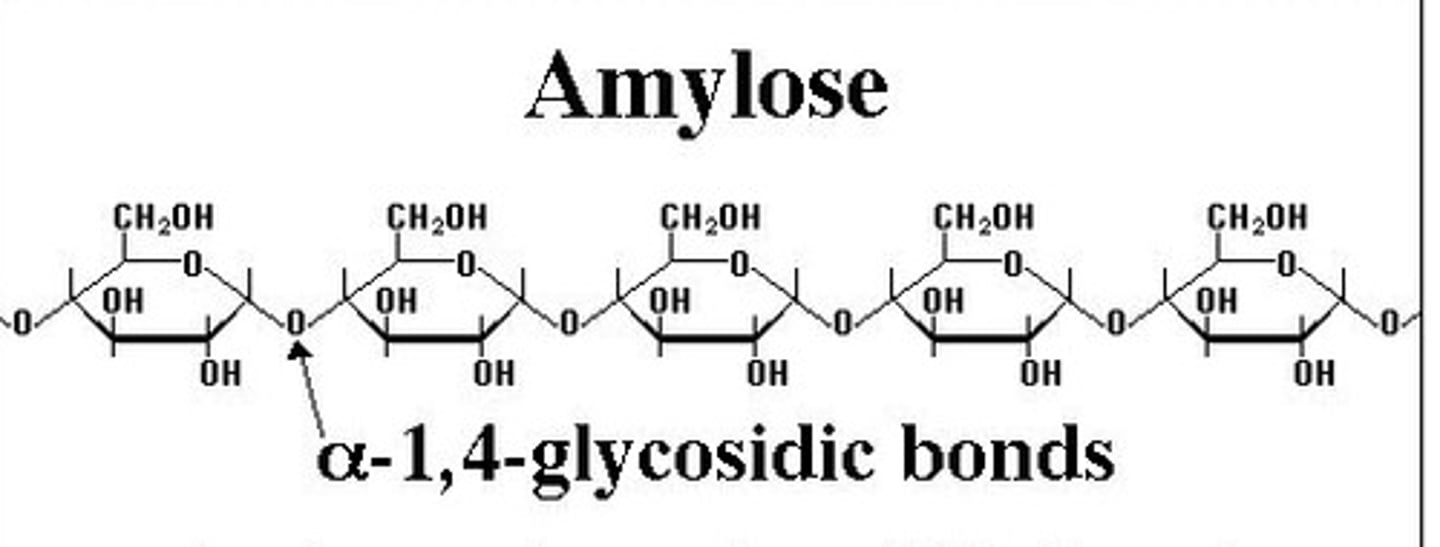
How is amylose adapted for its use in starch?
Coils make it compact for efficient storage of glucose
Structure of amylopectin
- Polysaccharide formed from glucose monomers joined together by 1-4 and 1-6 glycosidic bonds
- Long branched chain
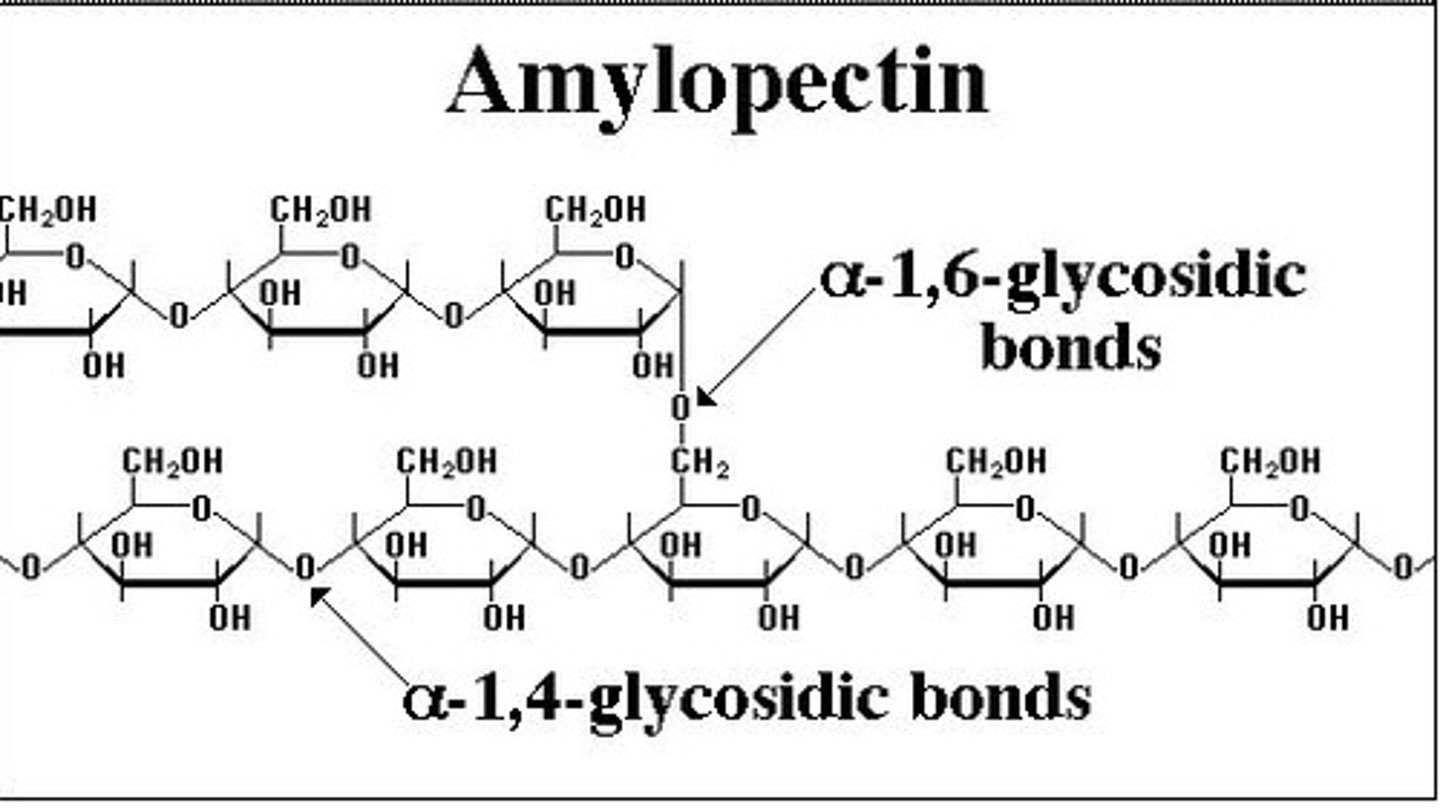
How is amylopectin adapted for its use in starch?
High surface area for efficient hydrolysis
Structure of glycogen
- Long branched chain
- made from condensation reaction of alpha glucose bonded together by 1-4 and 1-6 glycosidic bonds
Function of glycogen
Store carbohydrates in mammals
How is glycogen adapted for its function?
- lots of branches to increase surface area for efficient hydrolysis
- compact molecule for storage
What is the structure of cellulose?
- 1-4 glycosidic bonds between beta glucose where every 2nd beta glucose is inverted
- Forms a straight unbranched chain
What is a microfibril?
Hydrogen bonds attach each cellulose chain together to form thicker fibres called microfibrils
What is cellulose used for?
- Support cell wall
- Allows cells to become turgid
How is cellulose adapted for its function?
- Hydrogen bonds between the cellulose chain make the microfibrils very strong but still flexible to provide support
What is a triglyceride made of?
glycerol molecule + 3 fatty acid chains
What bonds are in a triglyceride?
Ester bonds between the 3 OH groups on the glycerol and the OH groups on the fattty acids formed by condensation reactions
What is a phospholipid made out of?
A glycerol molecule, a phosphate group and two fatty acid chains
What bonds are in a phospholipid?
Ester bond between 2 OH groups on the glycerol and the OH group of each fatty acid chain formed by condensation reactions
What is the head of a phospholipid?
Hydrophilic- attracted to water
What is the tail of a phospholipid?
Hydrophobic- repulsed by water
Properties of triglycerides
Fatty acid chains are hydrophobic making lipids insoluble in water
-> they bundle together as insoluble droplets as their tails face inwards and the glycerol heads shield them from water
Properties of phospholipids
- Phosphate group is hydrophilic and the fatty acid chains are hydrophobic allowing phospholipids to form bilayers which make up membranes around a cell
Uses of triglycerides
Energy stores- lots of energy released when the fatty acid chain is broken down
Uses of phospholipids
Membranes + hormones
Properties of saturated fats
- no double bonds
- maximum amount of hydrogen it can hold
- solid at room temperature ( high melting point)
Properties of unsaturated fatty acids
- Double bonds between carbon atoms
- Contain fewer hydrogen atoms
- Bends and kinks in chain
- lower boiling point
What is a phospholipid bilayer?
A double layer formed by phospholipids with their hydrophilic heads facing outwards and their hydrophobic tails facing inwards
How is a phospholipid bilayer adapted for its function?
- Centre of the bilayer is hydrophobic so water soluble substances cannot easily pass through
- This creates a barrier and allows separation of solutions allowing different conditions to be created either side of the membrane
What is protein made of?
Amino acids (monomers)
How many amino acids are there?
20
Structure of an amino acid
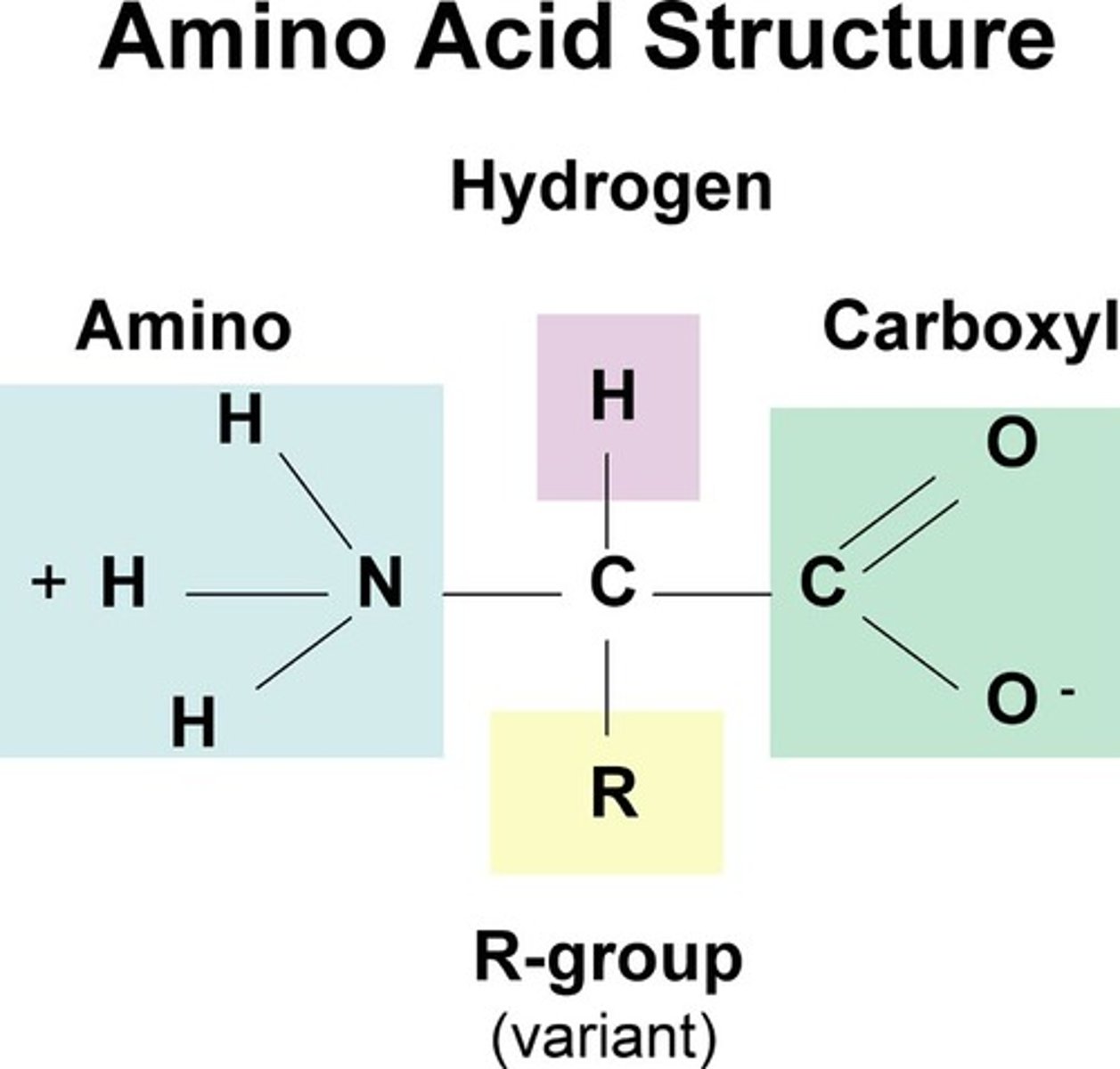
What bond forms between 2 amino acids?
Peptide bond ( -C-N-)
What is two amino acids joined together called?
Dipeptide
What are more than 2 amino acids joined together called?
Polypeptide
What is the primary structure of an amino acid?
Number and sequence of amino acids which can determine the 3D shape or tertiary structure
What is the secondary structure of a protein?
- Hydrogen bonds between amino acids in the chain
- Chain can coil into alpha helix
- Chain can fold into beta pleated sheets
What do the hydrogen bonds in the secondary structure of a protein mean?
Makes the structure stable
What are most channel proteins made of?
Alpha helices
What is the tertiary structure of a protein?
- 3D shape of the polypeptide chain
- creates a specific shape due to the sequence of amino acids
What bonds are in the tertiary structure of a protein?
- hydrogen bonds
- ionic bonds
- disulphide bridges
- van der Waals forces
What is the quaternary structure of a protein?
If proteins are made of more than one polypeptide chain then they are joined together to create a quaternary structure
What are examples of quaternary structures?
Antibodies + haemoglobin
Where are hydrogen bonds in the tertiary structure?
Between the OH group of one amino acid and the C=O group of another
Where are the disulphide bridges in the tertiary structure?
Form between sulfur-containing amino acids that are near each other
Where are the ionic and covalent bonds in the tertiary structure?
Between R groups
Where are van Der Waals forces in the tertiary structure?
Weak intermolecular forces that hold the tertiary structure in shape
What are the 2 types of proteins?
globular + fibrous
Characteristics of globular proteins
Soluble + spherical in shape
What are the characteristics of fibrous proteins?
- straight chain polypeptides
- insoluble
Structure of fibrous proteins
Straight chain polypeptides that are side by side and held together by hydrogen bonds
What are the benefits of fibrous proteins being insoluble?
More resistant to physical + chemical attack
What are the effects of changing temperature on proteins?
- Heat energy increases the kinetic energy of the molecule making it vibrate more
- The vibrations are strong enough to break the intermolecular forces + affect the shape of the protein
What are the effects of changing pH on proteins?
Extreme changes in pH can disrupt the ionic charges on the R groups, resulting in ionic bonds breaking
What is an enzyme?
A biological catalyst that speeds up chemical reactions
What is the function of an enzyme?
Catalyse specific metabolic reactions at a cellular and extracellular level without being used up in the reaction
What is the structure of an enzyme?
Proteins with a 3D structure
Specifically shaped active site that is complementary to a certain substrate
What is an enzyme-substrate complex?
When the enzyme binds to the substrate
How do enzymes speed up metabolic reactions?
Lowering the activation energy of a reaction:
- hold substrates close together, reducing repulsion and allowing them to bond more easily
- put more strain on bonds of a substrate allowing them to break apart more easily
What is the induced fit model?
The active site is not exactly complimentary to the substrate molecule. When the substrate collides with the enzyme the active site can change shape slightly to fit around the substrate and form an ES complex
How does enzyme concentration affect enzyme activity?
- Inc enzyme increases active sites available
- More ES complexes can form
- Rate of reaction increases until substrate concentration becomes the limiting factor
How does substrate concentration affect enzyme activity?
-Inc substrates inc rate of reaction
- more substrate available meaning more collisions
- more ES complexes can form
- Rate of reaction will slow as enzyme concentration becomes the limiting factor and all active sites are occupied
How does temperature affect enzyme activity?
As temperature increases so does the rate of reaction:
- more kinetic energy so molecules move faster increasing the number of collisions and ES complexes formed
What happens if the temperature exceeds the enzymes optimum temperature?
Enzyme molecule vibrates too much and the bonds which maintain the tertiary structure are broken -> active site changes shape and no more ES complexes can form as they enzyme is permanently damaged
How does pH affect enzyme activity?
Above and below the optimum pH pf an enzyme the H+ and OH- ions disrupt the ionic and hydrogen bonding that hold the tertiary structure in shape
-> site of the active site can change and no ES complexes can form and the enzyme is permanently denatured
What do competitive inhibitors do?
Have a similar shape to the substrate so can compete with the substrate and bind to the active site and block it -> prevent ES complexes from forming
What do non-competitive inhibitors do?
- Bind to the allosteric site
- causes the shape of the active site to change to it is no longer complimentary and ES complexes can't be formed
How does increasing substrate concentration affect competitive inhibitors?
Increasing substrate will reverse the effects of the competitive inhibitor as the substrate will out compete the inhibitor