Molecular Speeds
0.0(0)
Card Sorting
1/8
There's no tags or description
Looks like no tags are added yet.
Last updated 11:56 PM on 11/19/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
1
New cards
T1
(i)
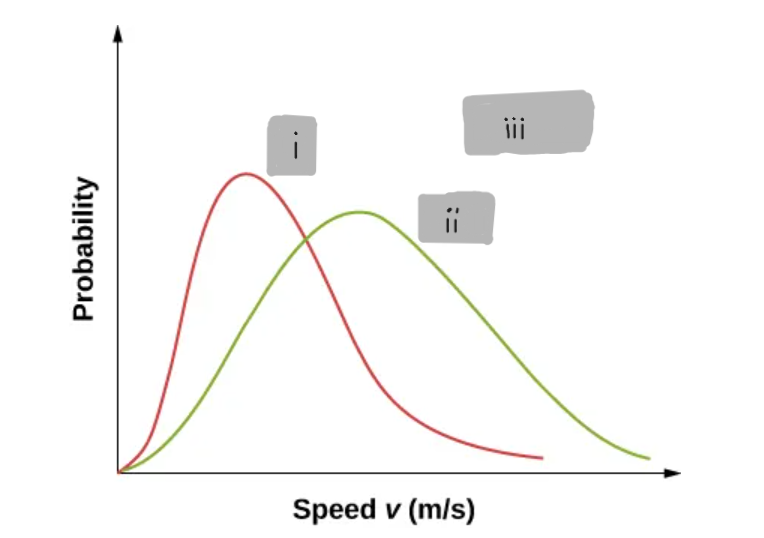
2
New cards
T2
(ii)
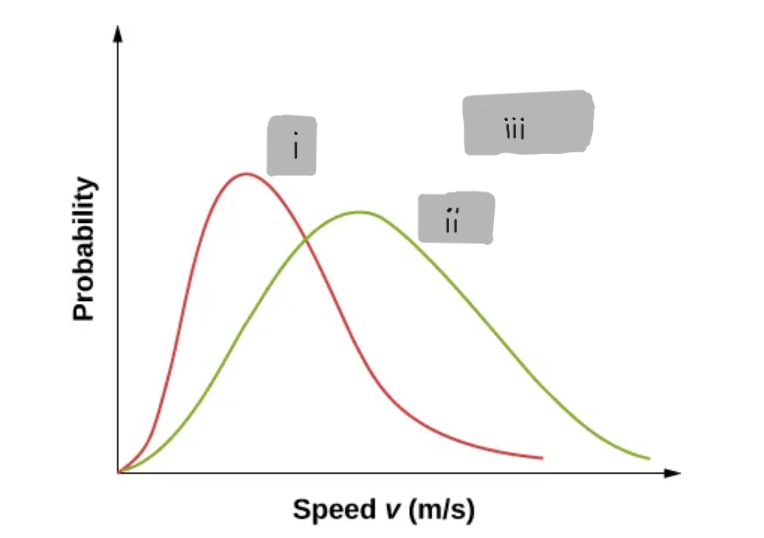
3
New cards
T2 > T1
(iii)
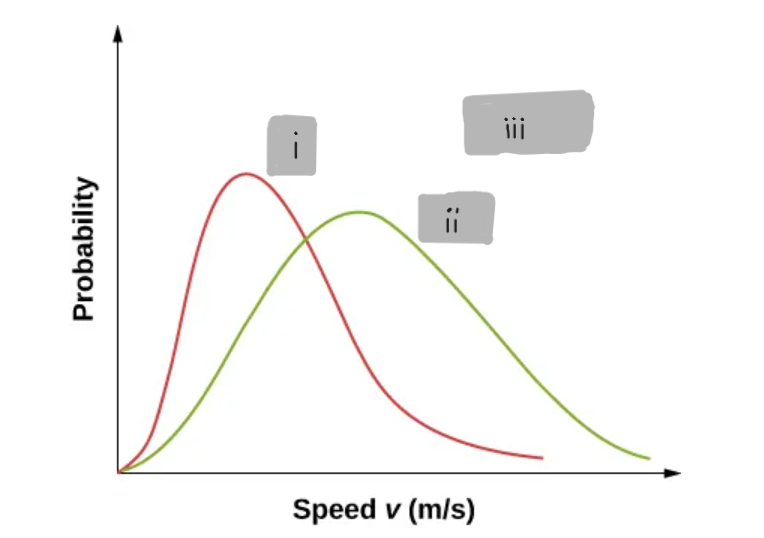
4
New cards
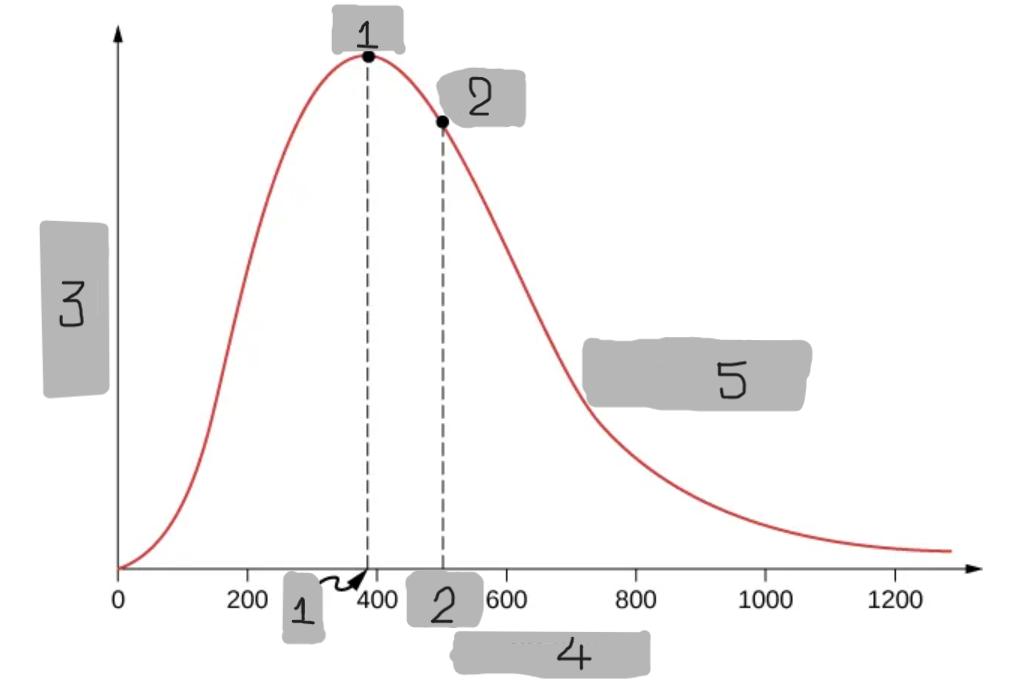
v_p
(1)
5
New cards
v_rms
(2)
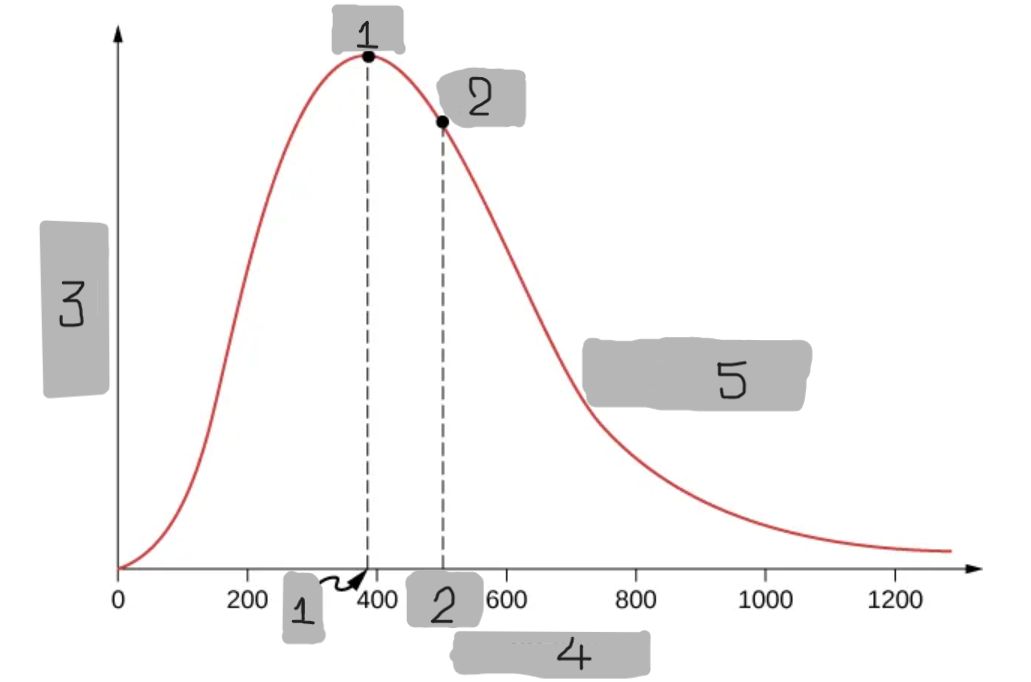
6
New cards
Probability
(3)
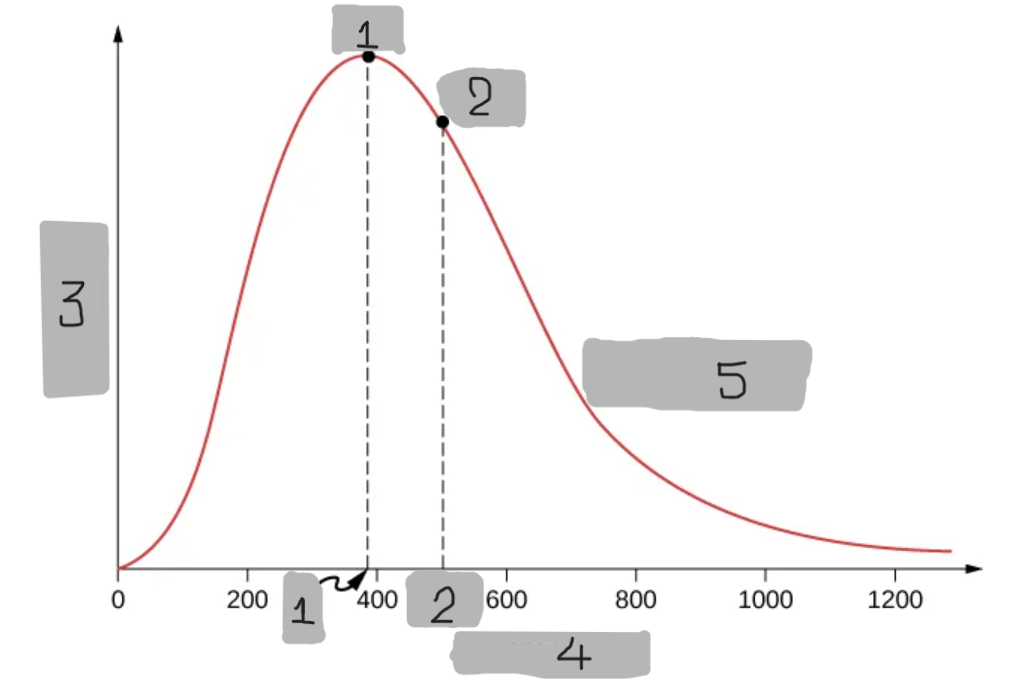
7
New cards
Speed v (m/s)
(4)
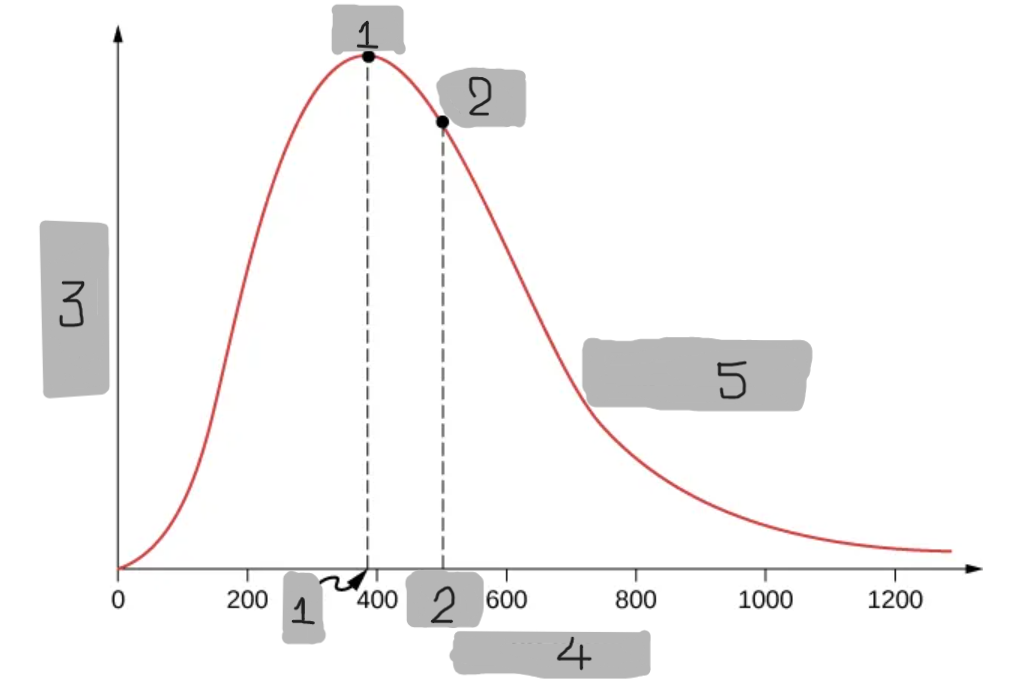
8
New cards
O2 at T = 300 K
(5)
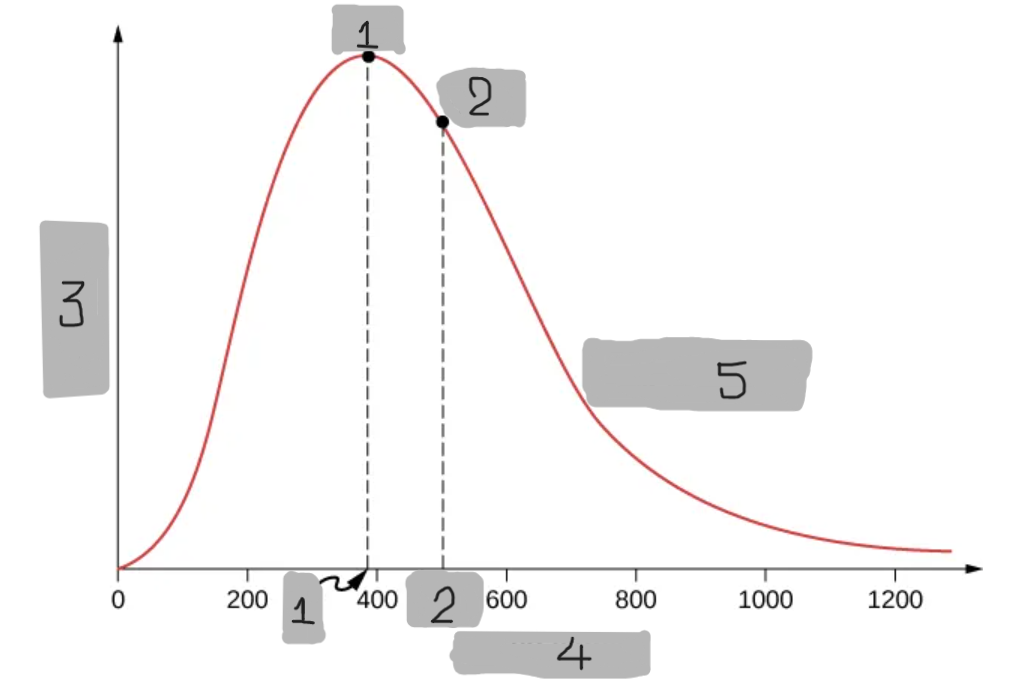
9
New cards
Maxwell-Boltzmann Distribution
A probability that describes how many molecules in an ideal gas move at each possible speed.
Predicts that most molecules have moderate speeds,
Very few move extremely fast, and some move very slow