Chapter 2: Alkanes and Cycloalkanes
1/130
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
131 Terms
hydrocarbons
compounds that only contain C and H atoms
4 types of hydrocarbons
alkanes - only carbon-carbon single bonds
Alkenes - Double Carbon and Carbon Bonds
Alkynes - Triple Carbon and Carbons Bonds
arenes/aromatic - Benzene -Like Rings
Are hydrocarbons polar or nonpolar?
very non-polar, so immiscible with water (hydrophobic)
structure of alkanes
saturated hydrocarbons, that is they contain the maximum number of H atoms possible for the number of C atoms present
Four Bonds for Carbon
Tetrahedral
Sp3 hybridized
generic formula for an alkane
- CnH2n+2
- This means every C atom:
is sp3 hybridized,
forms 4 single bonds,
has tetrahedral geometry, with
bond angles of ~109.5°
two forms of alkanes
normal (or linear) and branched
branches alkanes can have branches branches
how does the number of C's affect the number of constitutional isomers?
the number of possible constitutional isomers grows quickly as the number of C's increase.
linear alkane
branched alkane
carbon's ability to form strong C-C bonds
- creates a large number of possible isomers
- other elements do not form large molecules of like atoms covalently bonded together
- this is why carbon is uniquely able to form the large/complex molecules of life
Constitutional Isomers
Differ in the way the atoms are connected to each other
Same # of each element ( same molecular formula )
Different Structure ( Atoms are bonded differently to each other )

The IUPAC system
- a systematic method for the naming of compounds
- Allows anyone to draw a structure from the name, and vice versa.
-A compound's name is the same worldwide
The IUPAC name includes
-Parent name (longest carbon chain)
-Names of substituents
-Location of substituents
parent names for alkanes: C = 1
parent = meth
name of alkane = methane
parent names for alkanes: C = 2
parent = eth
name of alkane = ethane
parent names for alkanes: C = 3
parent = prop
name of alkane = propane
parent names for alkanes: C = 4
parent = but
name of alkane = butane
parent names for alkanes: C = 5
parent = pent
name of alkane = pentane
parent names for alkanes: C = 6
parent = hex
name of alkane = hexane
parent names for alkanes: C = 7
parent = hept
name of alkane = heptane
parent names for alkanes: C = 8
parent = oct
name of alkane = octane
parent names for alkanes: C = 9
parent = non
name of alkane = nonane
parent names for alkanes: C = 10
parent = dec
name of alkane = decane
Alkane names always end with
"ane" suffix
IUPAC naming first step
Identify the parent chain - the longest consecutive chain of carbons
If there is more than one possible parent chain,
choose the one with the most substituents attached.
If the parent chain is cyclic,
add the prefix "cyclo"
generic formula for cycloalkanes with one ring
CnHn
IUPAC naming second step
identify and name the substituents
Substituent
- Any part of the structure not included in the parent chain
-
When an alkane is a substituent,
The "ane" suffix is changed to "yl"
The first part of the substituent's name still identifies
how many C's are present.
names of alkyl groups: C = 1
methyl
names of alkyl groups: C = 2
ethyl
names of alkyl groups: C = 3
propyl
names of alkyl groups: C = 4
butyl
names of alkyl groups: C = 5
pentyl
names of alkyl groups: C = 6
hexyl
names of alkyl groups: C = 7
heptyl
names of alkyl groups: C = 8
octyl
names of alkyl groups: C = 9
nonyl
names of alkyl groups: C = 10
decyl
Carbons in the parent chain have to be
•numbered "Locant"
2-methylpentane
- means there is a methyl group on carbon #2 of the pentane chain.
- •All parts of the structure are included in its name
(Parent and substituents)
first guideline to follow when numbering the parent chain
- If ONE substituent is present, number the parent chain from the end that the puts the substituent on the lowest numbered carbon.
- There will never be a compound named 6-methylheptane
second guideline to follow when numbering the parent chain
When multiple substituents are present, number the parent chain to give the first substituent the lowest number possible
When 2 substituents are on the same carbon,
they both get their own number
third guideline to follow when numbering the parent chain
If there is a tie for the first substituent from both ends, then number the parent chain so that the second substituent from either end gets the lowest number possible
If there is no tie-breaker as just discussed,
then assign the lowest number to the carbon bearing the alphabetically first substituent
When halogens are substituents,
their names are abbreviated as
Fluoro
Bromo
Chloro
iodo
- ex: 1-bromo-5-chloropentane
To assemble the complete name
Put the # and name of each substituent before the parent chain name, in alphabetical order
if multiple substituents are identical,
- A prefix is used (di, tri, tetra, penta, etc.)
- These multiplier prefixes are ignored when alphabetizing the substituents
IUPAC Rules - Summary
1.Identify the parent chain
2.Identify and Name the substituents
3.Number the parent chain; assign a locant to each substituent
4.List the numbered substituents before the parent name in alphabetical order ( if tie for number for substituents name for alphabetical order)
A -CH3 group is a
methyl group
nature of carbon-carbon bonds
-an- ( all single bonds )
-en- ( one or more double bonds)
-yn- (one or more triple bonds)
Hydrocarbon
-e-
alcohol
-ol
aldehyde
-al
amine
-amine;
ketone
-one
carboxylic acid
-oic acid
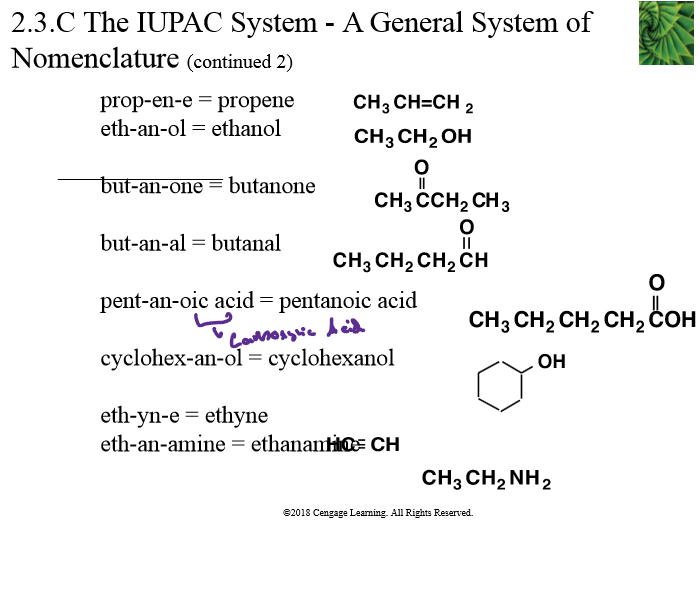
Examples of IUPAC System
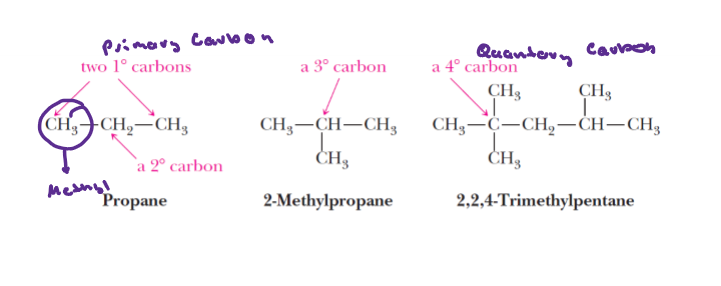
Quaternary
Carbons bonded to four other carbon atoms
The strength of IMF's between molecules is also dependent on
the overall shape of the molecule
Solubility, the ability to dissolve into a solvent is dependent
on IMFs
Boiling points and melting points are affected by
IMF's, and also on the mass of the molecules.
3 types of IMFs, by decreasing strength they are:
1)Hydrogen bonding
2)Dipole-dipole
3)Van der Waals or London Dispersion
IMFs of alkanes
Alkanes only have van Der waals/London dispersion forces because C-C and C-H bonds are non-polar
Van der Waals or (London) dispersion forces
- arise from the movement of electrons within a molecule.
- This natural motion can produce an uneven distribution of the electrons (polarization of the distribution) resulting in a temporary dipole moment in the molecule.
- This will induce the movement of electrons in adjacent molecules producing a dipole moment in them as well.
"induced" dipole moments
are very brief, as they disappear when the electrons move to new locations within the molecule, so these forces are very brief and weak, only 2-5 kJ/mol
Since the only intermolecular forces alkanes have is van der Waals interactions,
they have the lowest melting and boiling points compared to other types of organic molecules
Do not confuse weak intermolecular forces with intramolecular covalent bonds.
Intramolecular covalent bonds in alkanes are very strong.
The strength of the IMFs also depend on
the amount of contact between the molecules, especially for dispersion forces.
shape of the molecule can affect the surface area of contact
Long thin molecules have more surface in contact with each other than spherical molecules.
When predicting the relative bp's or mp's of alkanes:
1.First compare MW's : Higher MW = Higher bp or mp
2.Second: If MW's are nearly the same, compare molecular shape
Longer chain length = higher bp or mp
MW has a much bigger effect on
bp and mp than chain length
Conformational isomers
- are compounds with atoms bonded in the same order, but the atoms are located in different places in space due to bond rotations
- This is achieved by rotating about C-C single (s) bonds or the dihedral (or torsion) angle (q).
- ("rotamers" or "conformers")
only differ by rotating the bonds of the molecule
Conformations are best viewed by
- by looking down the length of a bond
- This view can be represented by a "Newman projection."
"Newman projection."
- What becomes clearly visible is the "dihedral angle" or "torsion angle"
- staggered and eclipsed conformations
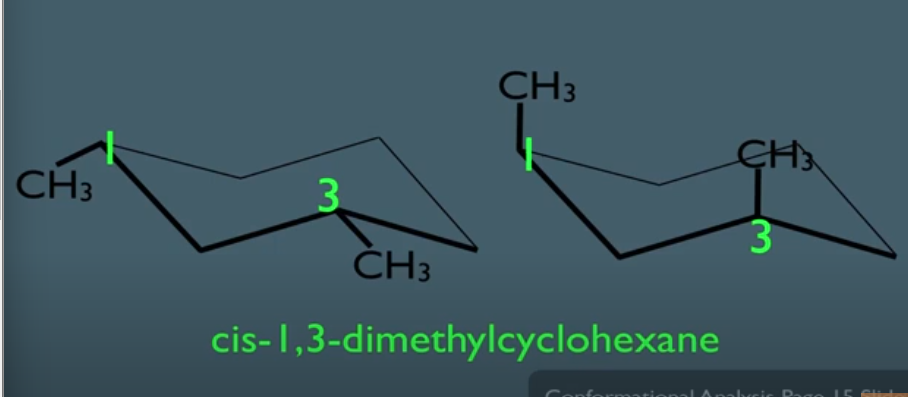
staggered conformation
- Dihedral angle = 60
- lowest in energy
- where molecules spend most of their time because most stable state
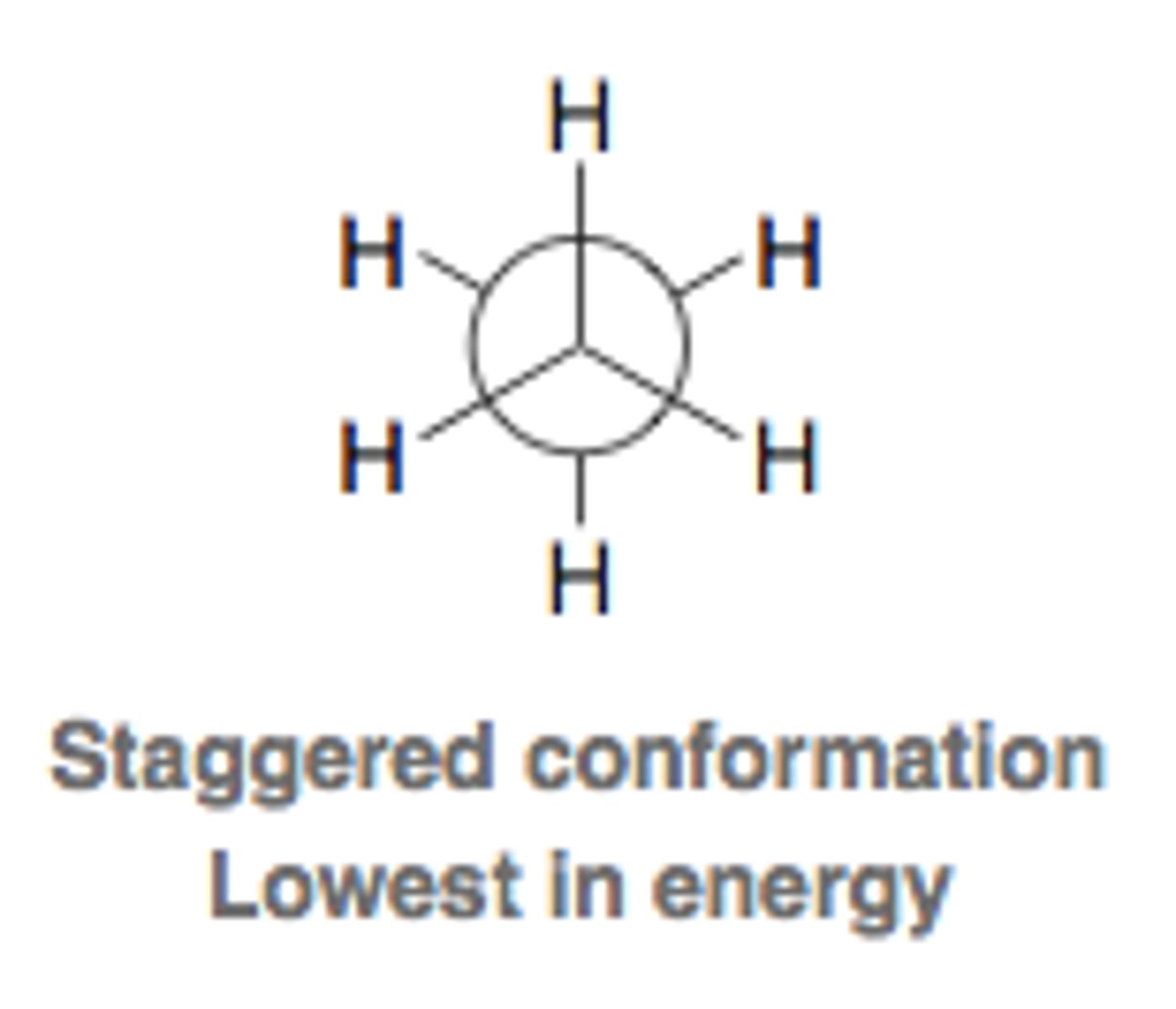
eclipsed conformation
- dihedral angle = 0
- highest in energy
- where molecules spend the least of their time because most unstable state
In ethane there are two extremes that represent high and low energy "conformations" of ethane.
- As one of the carbons is rotated though a full 360o circle, three eclipsed conformations and three staggered conformations will be encountered.
- The internal energy of the molecule reaches a maximum in the eclipsed conformations, and a minimum in the staggered conformations.
The difference in energy is caused by
"steric strain" between the H atoms
"Strain:"
is just what it sounds like, internal stress in a molecule
"Steric:"
is used in organic chemistry to describe a situation when atoms are being forced so close together that the repulsion between their electron clouds is increased.
"steric strain:"
caused when atoms are forced close together
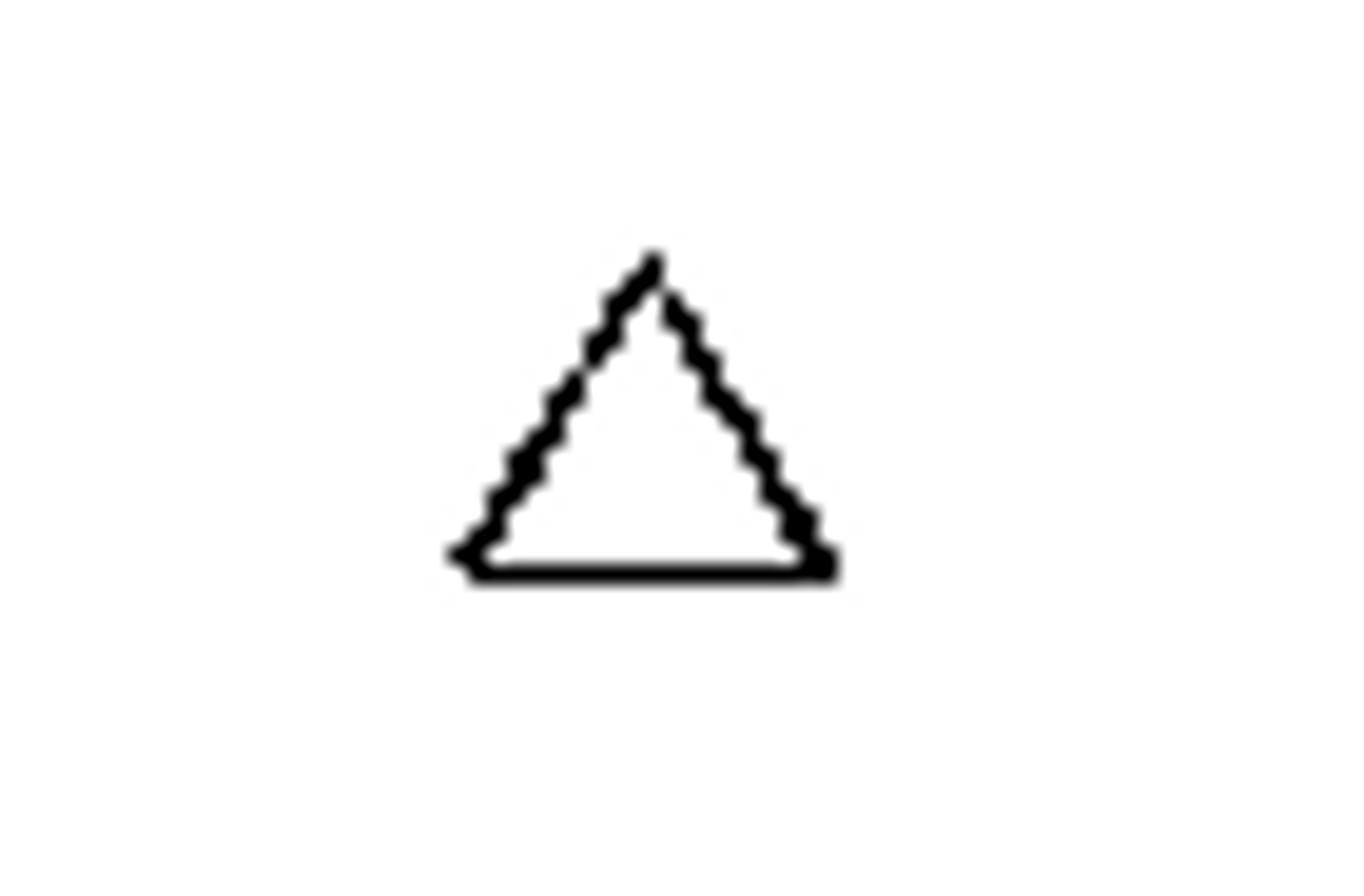
cyclopropane
- smallest ring structure possible
-It is rigid and very highly strained as the bond angles (60°) are very distorted from the ideal (109.5°)
-It is more reactive than a linear alkane as the strained C-C bonds are easier to break
-The 3 carbon atoms all lie in one plane
cyclopropane structure
"angle strain" or "ring strain"
results when bond angles are forced away from their most stable angles
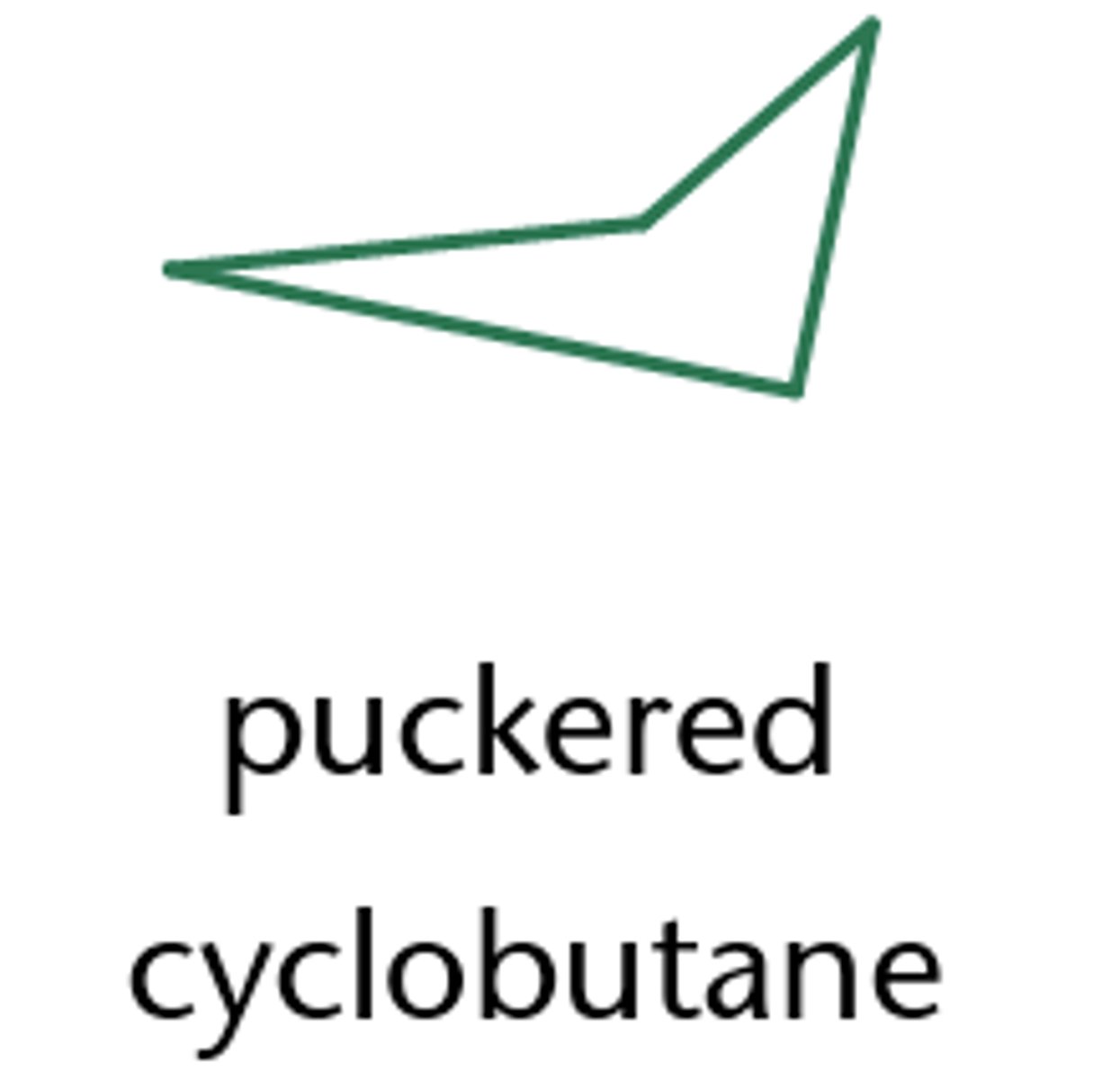
cyclobutane
- "puckered" conformation"
-It is more slightly more flexible than cyclopropane and is not flat. It adopts a "puckered" conformation which reduces the amount of steric and angle strain.
- It is more reactive than a linear alkane as the strained C-C bonds are easier to break, bond angle ~90°
cyclobutane structure
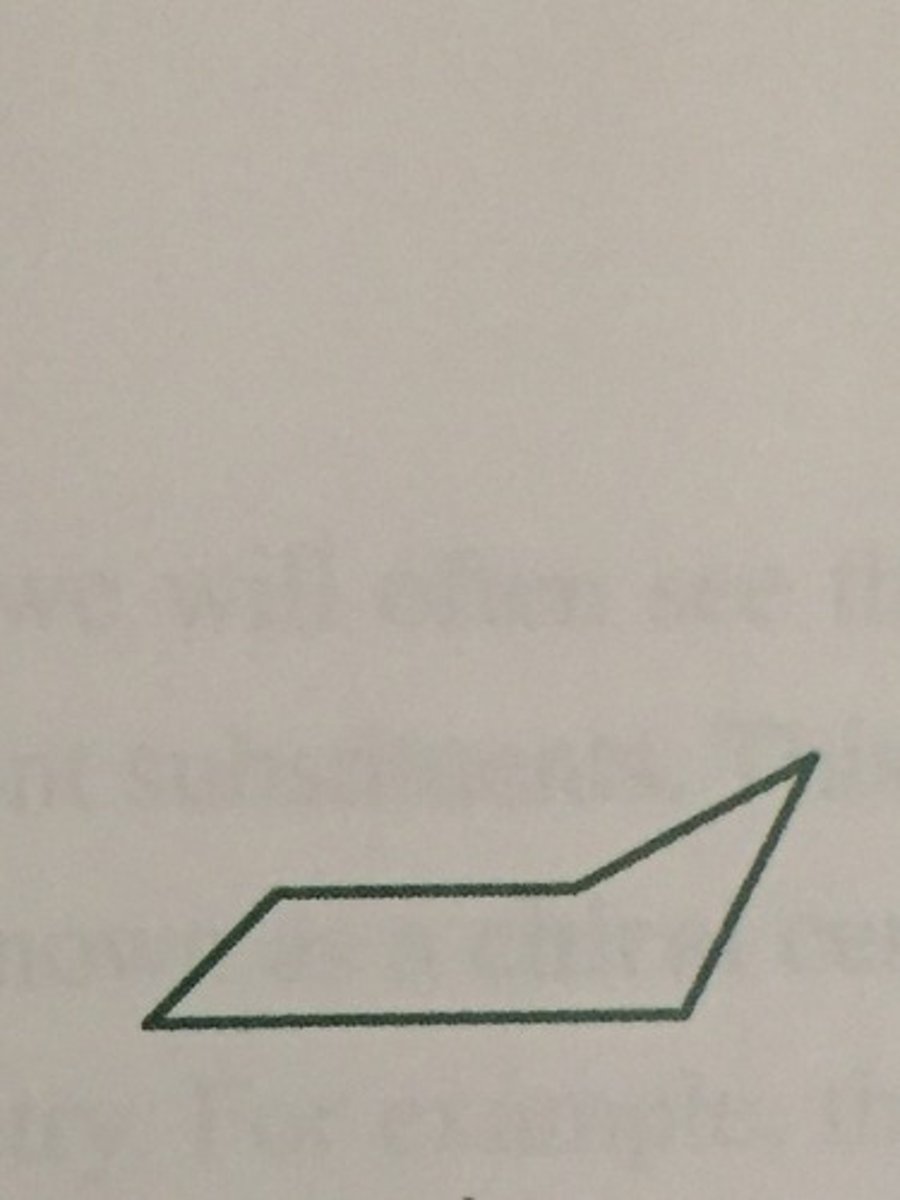
Cyclopentane
- most stable in an "envelope" conformation
- It is more flexible than cyclobutane and the bond angles are ~105° in the envelope conformation, which closer to 109.5o
cyclopentane structure
Cyclohexane
- most stable in a "chair" conformation
- It is highly flexible and can adopt a strain free, non-planar, chair conformation with bond angles of 109.5°
- most common ring size in organic chemistry
cyclohexane structure
- Although we will draw it most often as a hexagon,
you must remember it is not flat.
- From the side it looks like this
- If you tilt it a little more, you can see where the chair name comes from.
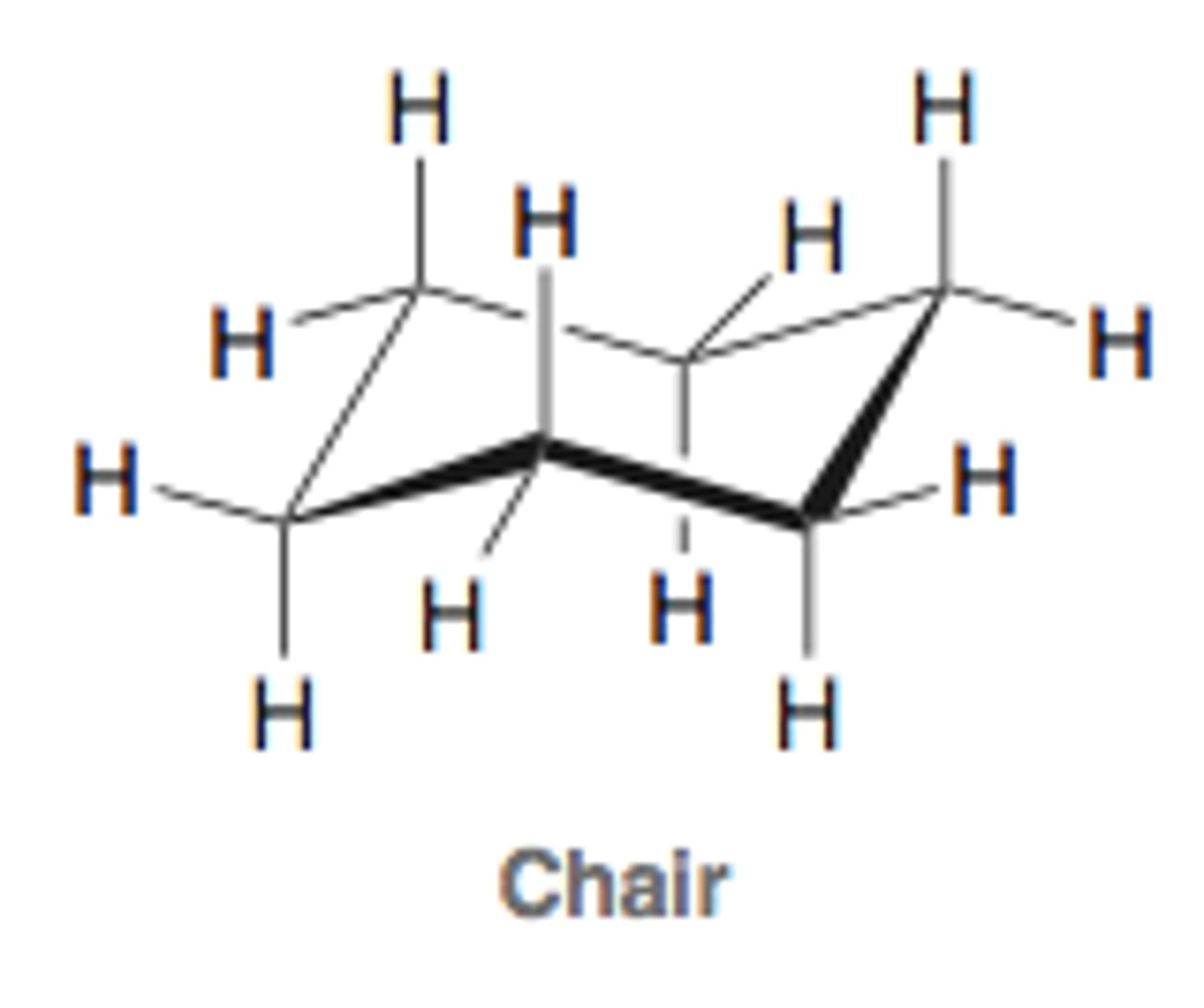
cyclohexane chair conformation
- most stable conformation of cyclohexane
- the bond angles are all ~109.5°.
(As opposed to the 120° that would result from planar hexagon.)
- Also, all bonds are in staggered conformations.
- This conformation creates two distinct subsets of hydrogen atoms.
cyclohexane subsets of hydrogen atoms
Six H's almost in the same plane as the C atoms (equatorial) and Six H's above and below that plane (axial)
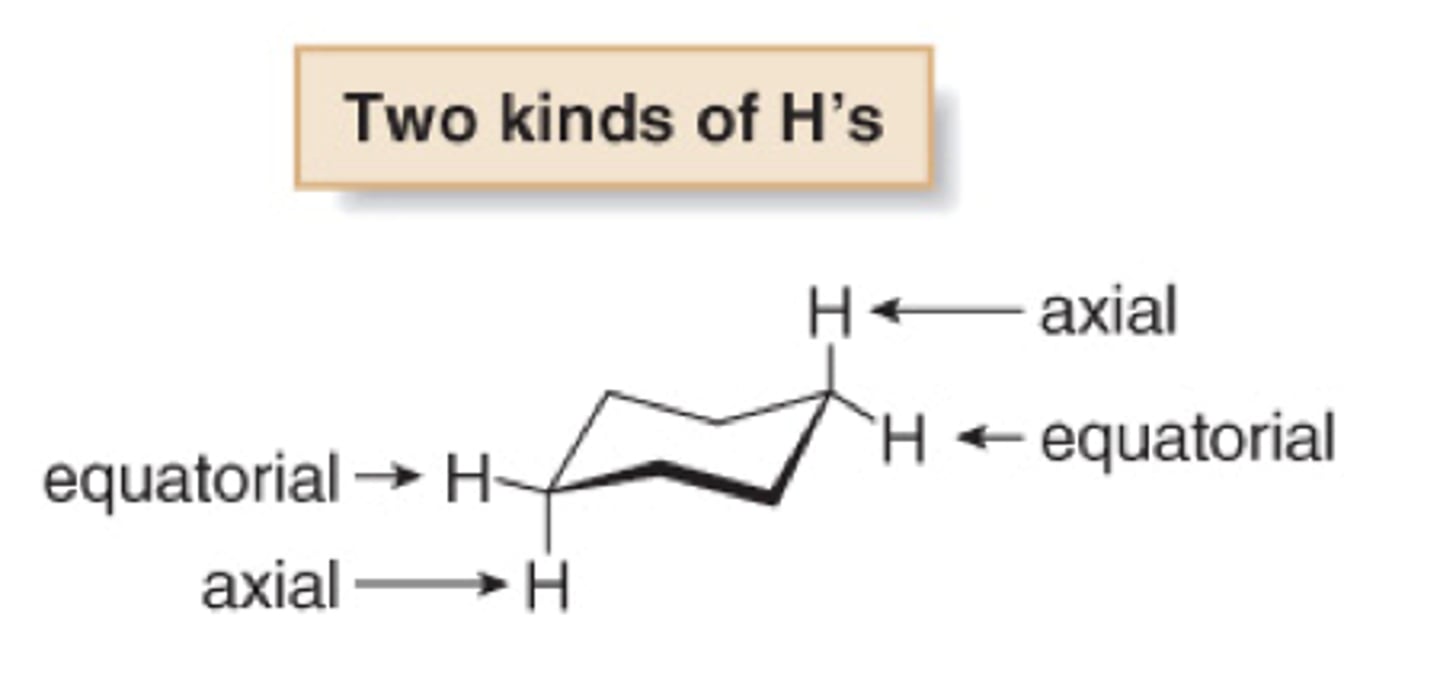
cyclohexane bonds
Since the C-C bonds are all single (s) bonds, it is possible to rotate about these bonds.
ring inversion or a "chair-chair" flip
all equatorial H become axial and axial become equatorial
are other conformations possible for cyclohexane beside chair?
- Other conformations of cyclohexane are possible, but they are higher in energy and less stable.
- One example is the "boat" conformation.